Abstract
Prothrombinase activates prothrombin through initial cleavage at Arg320 followed by cleavage at Arg271. This pathway is characterized by the generation of an enzymatically active, transient intermediate, meizothrombin, that has increased chromogenic substrate activity but poor clotting activity. The heavy chain of factor Va contains an acidic region at the COOH terminus (residues 680−709). We have shown that a pentapeptide from this region (DYDYQ) inhibits prothrombin activation by prothrombinase by inhibiting meizothrombin generation. To ascertain the function of these regions, we have created a mutant recombinant factor V molecule that is missing the last 30 amino acids from the heavy chain (factor VΔ680−709) and a mutant molecule with the 695DYDY698 → AAAA substitutions (factor V4A). The clotting activities of both recombinant mutant factor Va molecules were impaired compared to the clotting activity of wild-type factor Va (factor VaWt). Using an assay employing purified reagents, we found that prothrombinase assembled with factor VaΔ680−709 displayed an ∼39% increase in kcat, while prothrombinase assembled with factor Va4A exhibited an ∼20% increase in kcat for the activation of prothrombin as compared to prothrombinase assembled with factor VaWt. Gel electrophoresis analyzing prothrombin activation by prothrombinase assembled with the mutant molecules revealed a delay in prothrombin activation with persistence of meizothrombin. Our data demonstrate that the COOH-terminal region of factor Va heavy chain is indeed crucial for coordinated prothrombin activation by prothrombinase because it regulates meizothrombin cleavage at Arg271 and suggest that this portion of factor Va is partially responsible for the enhanced procoagulant function of prothrombinase.
Blood coagulation is initiated at the site of vascular injury and results in the activation of prothrombin to α-thrombin by the prothrombinase complex. Prothrombinase is composed of the enzyme factor Xa bound to its cofactor, factor Va, on a phospholipid surface in the presence of Ca2+(1). Prothrombin and α-thrombin have two distinct exosites (anion binding exosite I, ABE-I,1 and anion binding exosite II, ABE-II) that are responsible for the functions of the molecules. The role of (pro)exosite I of prothrombin within prothrombinase is dependent on the incorporation of factor Va into the complex (2,3). Two pathways for prothrombin activation are possible: membrane-bound factor Xa alone activates prothrombin following initial cleavage at Arg271 followed by cleavage at Arg320, while the fully assembled prothrombinase complex activates prothrombin following the opposite pathway, initial cleavage at Arg320 followed by cleavage at Arg2714–8. Activation of prothrombin via this latter pathway is characterized by the generation of an intermediate, meizothrombin, that has proteolytic activity and results in a significant increase in the catalytic efficiency of factor Xa with respect to α-thrombin formation (9).
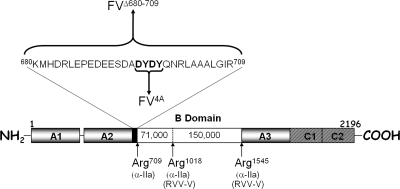
Human factor V circulates in plasma as a 330000 molecular weight, single-chain protein that consists of three domains in the order A1−A2−B−A3−C1−C2. Proteolytic cleavage of the cofactor by α-thrombin occurs sequentially at Arg709, Arg1018, and Arg1545 to produce a heterodimer consisting of a heavy chain (Mr ∼ 105000) and a light chain (Mr ∼ 74000) associated via divalent metal ions (10–12) (Figure 1). The heavy chain of the cofactor contains an acidic amino acid region that has been shown to be important for cofactor function (amino acids 680−709) (13). Earlier data have suggested that factor Va interacts with meizothrombin (14) and that this acidic region is implicated in the productive interaction of factor Va with prothrombin (15–17). Various proteases can cleave this acidic region to produce a cofactor with a truncated heavy chain (18–21). Gerads et al. first showed that it is possible to selectively eliminate the acidic region from factor Va heavy chain by an enzyme purified from the venom of the snake Naja naja oxiana(18). Using proteins of bovine origin, the authors demonstrated that while elimination of the COOH-terminal region of factor Va results in a molecule with severely impaired clotting activity, incorporation of the truncated cofactor molecule into prothrombinase resulted in an increased kcat for the activation of prothrombin (18). The same authors also showed that cleavage of the human factor Va heavy chain by the same enzyme results in a cofactor with impaired clotting activity (18). Subsequently, Bakker et al. using the same purified enzyme identified the cleavage site of the enzyme at His682 in the human factor Va heavy chain [this amino acid is conserved in the bovine cofactor (22,23)] and demonstrated that prothrombinase assembled with a human factor Va molecule missing the Asp683−Arg709 portion has an increased kcat for the activation of prothrombin as compared to the kcat of prothrombinase assembled with the plasma cofactor (19). Afterward, Camire et al. using cathepsin G (CG) and human neutrophil elastase demonstrated that prothrombinase assembled with factor Va molecules missing the COOH-terminal domain of the heavy chain results in enzymes that consistently express higher kcat values, suggesting that these molecules are “more active” cofactors than purified plasma factor Va activated with α-thrombin in an assay using purified reagents (20). Surprisingly, and in line with the initial findings of Gerads et al. (18), the same factor Va molecules exhibited a significant decrease in clotting activity (20). More recently, using a purified enzyme from the snake venom of Naja nigricollis nigricollis, we have also shown that a factor Va molecule missing a portion of the COOH terminus of the heavy chain has decreased clotting activity (21). Altogether, these studies reveal that removal of the acidic COOH-terminal portion of factor Va heavy chain results in a cofactor molecule that is deficient in its clotting activity. However, prothrombinase assembled with these truncated cofactor molecules produces significantly higher kcat values for prothrombin activation when assessed in assays using purified reagents and a chromogenic substrate specific for α-thrombin. A molecular explanation for these paradoxical observations has not yet been provided.
Figure 1.
Mutants of human factor V. Factor V is activated following three sequential cleavages by α-thrombin at Arg709, Arg1018, and Arg1545. These cleavages release the active cofactor composed of heavy and light chains associated in the presence of divalent metal ions, and two activation fragments. The COOH terminus of the heavy chain contains an acidic hirudin-like amino acid region that is important for cofactor function. The mutations (deletions and point mutations) within the heavy chain are indicated together with the designation for the recombinant mutant factor V molecules created and used throughout the manuscript.
Approximately 35 years ago, Heldebrant and Mann first demonstrated that incubation of fragment 2 with α-thrombin results in a significant increase in the esterolytic activity of the enzyme toward Tos-l-Arg-OMe (TAME) as compared to the activity of α-thrombin alone toward the same substrate (24). This increase in the esterase activity of α-thrombin was concomitant with a decrease in its clotting activity (24). Subsequently, Franza et al. (25), Morita et al. (26), and Kornalik and Blombäck (27) reported that meizothrombin (and meizothrombin desfragment 1) generated by a component purified from the venom of Echis carinatus has poor clotting activity and high esterolytic activity toward TAME. Franza et al. specifically demonstrated that the meizothrombin species generated by E. carinatus has esterolytic activity toward TAME ∼30% higher than that of α-thrombin (25). Concomitantly, Myrmel et al. provided concrete evidence demonstrating that addition of fragment 2 to α-thrombin increases significantly the catalytic efficiency of the enzyme toward TAME, compared to the efficiency of α-thrombin alone against the same substrate (28). All these findings were later corroborated by two independent studies using either TAME or S-2238 (29–31), and it was hypothesized that there may be subtle but significant differences between the active sites of α-thrombin and meizothrombin (29). This conclusion was strengthened by earlier findings demonstrating that while DAPA, a specific noncovalent active site inhibitor of α-thrombin, interacts with prethrombin 2 with an affinity that is ∼30 times lower than that for α-thrombin, the inhibitor does not bind prothrombin and prethrombin 1 (32), and by data obtained using electron spin resonance to probe the active sites of meizothrombin and α-thrombin (33). Collectively, the findings suggest an important effect of cleavage at Arg271 on the progressive formation of the active site of α-thrombin, resulting in significant conformational differences between the active sites of α-thrombin and meizothrombin. More recently, structural data obtained from the only crystal structure of meizothrombin available in the literature [bovine meizothrombin desfragment 1 (34)] have established that the molecule is impaired in its clotting activity because it has not yet exposed ABE-II (which is covered by fragment 2). However, it is important to note that comparison of the structural data of various α-thrombin molecules provided in the literature with the crystal structure of meizothrombin (34) does not allow for a definite conclusion about the differences between the active site conformations of the two enzymes.
We have recently used overlapping peptides from region 680−709 of the factor Va molecule to show that an acidic pentapeptide with the sequence DYDYQ inhibits prothrombin activation by prothrombinase in a competitive manner with respect to substrate (21,35). We have further demonstrated that DYDYQ inhibits prothrombinase activity by inhibiting meizothrombin generation (36). Using data obtained with recombinant proteins, Toso and Camire have recently suggested that the COOH-terminal region of the factor Va heavy chain has no detectable effect on prothrombinase function (37). This conclusion was surprising and inconsistent with their findings since their data showed that (1) prothrombinase assembled with recombinant factor Va molecules missing a portion or the entire hirudin-like carboxyl-terminal end of the heavy chain (amino acid residues 678–709) had an increased kcat for the activation of prothrombin (from 129 to 150%) compared to prothrombinase assembled with the wild-type molecule in an assay using purified reagents and a chromogenic substrate specific for α-thrombin; (2) initial velocity measurements using the same assay demonstrated a 20−25% increase in the rate of α-thrombin formation by prothrombinase assembled with the same recombinant mutant cofactor molecules that were truncated at their carboxyl-terminal end; and (3) a recombinant factor Va molecule that is missing 17 amino acids from the carboxy-terminal portion of the heavy chain had decreased clotting activity (37). All these data are in complete accord with all earlier findings using plasma-derived factor Va molecules truncated at the carboxy-terminal end of their heavy chain (18–21) and demonstrate a crucial but yet undetermined contribution of the acidic COOH-terminal region of the heavy chain of the cofactor to prothrombinase activity during prothrombin activation.
It has been well established that while meizothrombin has poor clotting activity, its amidolytic activity is increased compared to that of α-thrombin toward small fluorescent and chromogenic substrates specifically used to assess α-thrombin activity (29–31,38). A logical hypothesis for reconciling all the findings described above is that the acidic COOH terminus of factor Va heavy chain, and more precisely the sequence DYDYQ, regulates meizothrombin concentration during the factor Xa-catalyzed prothrombin activation by prothrombinase. Thus, activation of prothrombin by prothrombinase assembled with a cofactor that is missing the acidic region will result in enhanced and stable meizothrombin production. This result will be translated by a factor Va molecule that is deficient in its clotting activity but produces an increase in kcat when introduced into prothrombinase. In contrast, in the presence of an excess of the acidic region [represented by DYDYQ (21,35,36)], no meizothrombin is produced by prothrombinase, resulting in the generation of α-thrombin through the alternative pathway characterized by initial cleavage of prothrombin at Arg271 and transient formation of prethrombin 2. This work was undertaken to test these hypotheses and to elucidate the role of the acidic COOH-terminal portion of the factor Va heavy chain during activation of prothrombin by prothrombinase.
Experimental Procedures
Materials, Reagents, and Proteins
Diisopropyl fluorophosphate (DFP), O-phenylenediamine dihydrochloride (OPD), N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (Hepes), Trizma (Tris base), and Coomassie Blue R-250 were purchased from Sigma (St. Louis, MO). Factor V-deficient plasma was from Research Proteins Inc. (Essex Junction VT). l-α-Phosphatidylserine (PS) and l-α-phosphatidylcholine (PC) were from Avanti Polar Lipids (Alabaster, AL). Normal reference plasma and the chromogenic substrate H-d-hexahydrotyrosol-alanyl-arginyl-p-nitroanilide diacetate (Spectrozyme-TH) were purchased from American Diagnostica Inc. (Greenwich, CT). H-d-Phenylalanyl-l-pipecolyl-l-arginyl-p-nitroaniline dihydrochloride (Chromogenix, S-2238) was purchased from Diapharma Group, Inc. (West Chester, OH). RecombiPlasTin for the clotting assays was purchased from Instrumentation Laboratory Co. (Lexington, MA). The reversible fluorescent α-thrombin inhibitor dansylarginine N,N-(3-ethyl-1,5-pentanediyl)amide (DAPA), a polyclonal antibody against factor V made in sheep, human prothrombin, RVV-V activator, human α-thrombin, and active site-blocked human meizothrombin [obtained following digestion of prothrombin with the purified component from the venom of the snake E. carinatus as described previously (30), FPR-meizothrombin] were from Haematologic Technologies Inc. (Essex Junction, VT). Human factor Xa was from Enzyme Research Laboratories (South Bend, IN). Human cathepsin G was from Calbiochem (EMD Chemicals, Inc., San Diego, CA). All molecular biology and tissue culture reagents and medium were from Gibco, Invitrogen Corp. (Grand Island, NY). Human plasma factor V was purified and concentrated using methodologies previously described employing the monoclonal antibody αhFV#1 coupled to Sepharose (39). Digestion of factor V by α-thrombin and cathepsin G (to obtain factor VaIIa/CG) or by α-thrombin and the purified enzyme from the venom of N. nigricollis nigricollis (factor VaIIa/NN) were performed as described previously (20,21). The clotting activities of all factor Va preparations were measured by a clotting assay using factor V-deficient plasma and standardized to the percentage of control as described previously (39,40) using an automated coagulation analyzer (START-4, Diagnostica Stago, Parsippany, NJ). Recombinant wild-type prothrombin and prothrombin rMZ-II that has only one cleavage site for factor Xa (i.e., Arg320) were prepared and purified as previously described (41). Phospholipid vesicles composed of 75% PC and 25% PS (termed PCPS vesicles throughout) were prepared as previously described (42).
Construction of Recombinant FV Molecules
Mutant factor V with the 695DYDY698 → AAAA substitutions (factor V4A) was constructed using the QuickChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions with the following primers (underlined nucleotides represent the mismatch): 5′-GATGAAGAGAGTGATGCTGCCGCTGCTGCCCAGAACAGA-3′ (sense) and 5′-TCTGTTCTGGGCAGCAGCGGCAGCATCACTCTCTTCATC-3′ (antisense). The deletion mutant (factor VΔ680−709) was constructed with the same kit. Primers for factor VΔ680−709 were 5′-CCTCCAGAATCTACAGTCATGGCTACACGGTCATTCCGAAACTCATCATTGAATCAGG-3′ (sense) and 5′-CCTGATTCAATGATGAGTTTCGGAACGACCGTGTAGCCATGACTGTAGATTCTGGAGG-3′ (antisense). PCR products were transformed into competent Escherichia coli cells, and positive ampicillin-resistant clones were selected. Before transfection, all mutant constructs were verified following sequencing in the Cleveland State University DNA Analysis Facility using a Beckman Coulter CEQ 8000 Genetic Analysis System (Beckman, Fullerton, CA) with factor V sequence-specific primers. The wild-type pMT2-FV and mutant pMT2-FV plasmids were isolated from the bacterial culture by the QIAfilter High Speed plasmid Midi Kit (Qiagen Inc., Valencia, CA).
Expression of Recombinant Wild-Type and Mutant Factor V in Mammalian Cells
COS-7L (Invitrogen) and COS-7 cells (American type Tissue Collection, ATCC, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics (100 μg/mL streptomycin and 100 IU/mL penicillin) in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Purified factor VWt, factor VΔ680−709, and factor V4A plasmids were transfected into the cells as described using FUGENE 6 (Roche Diagnostics, Basel, Switzerland) (43). Purification of all recombinant factor V molecules was performed as described previously (44). The concentration of all molecules was determined by an ELISA as detailed previously (43). The activity and integrity of the recombinant molecules were verified before and after activation with RVV-V activator or α-thrombin by clotting assays using factor V-deficient plasma and by sodium dodecyl sulfate−polyacrylamide gel electrophoresis (SDS−PAGE) followed by Western blotting using monoclonal and polyclonal antibodies. Full activation of the cofactor molecules prior to use in various experiments was ascertained by immunoblotting with both a mixture of monoclonal antibodies to factor V heavy and light chains (provided by K. G. Mann from the Department of Biochemistry, University of Vermont, Burlington, VT) and a polyclonal antibody to factor V made in sheep.
Analysis of Prothrombin Activation and FPR-Meizothrombin Cleavage at Arg271 by Gel Electrophoresis
Prothrombin (1.4 μM) was incubated with PCPS vesicles (20 μM), DAPA (50 μM), and factor Va (10−30 nM) in a buffer composed of 5 mM Ca2+ in 20 mM Tris and 0.15 M NaCl (pH 7.4). The reaction was initiated with the addition of factor Xa (0.5−1 nM) at room temperature over a 1 h time course. Aliquots (50 μL) from the reaction were removed at selected time intervals (as indicated in the figure legends) treated as described previously (36) and analyzed using 9.5% SDS−PAGE. Prothrombin and prothrombin-derived fragments were visualized by Coomassie Blue staining. Scanning densitometry, calculation of the rates of prothrombin consumption, and normalization to the prothrombin concentration were performed as described previously (36,45,46). FPR-meizothrombin cleavage at Arg271 was assessed in a similar manner using 12% SDS−PAGE.
Gel Electrophoresis and Western Blotting
SDS−PAGE analyses of recombinant proteins following activation were performed using 5 to 15% gradient gels according to the method of Laemmli (47). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes according to the method described by Towbin et al. (48). After transfer to PVDF membranes, factor Va heavy and light chain(s) were detected using the appropriate monoclonal and polyclonal antibodies (40). Immunoreactive fragments were visualized with chemiluminescence.
Measurement of Rates of α-Thrombin Formation in a Prothrombinase Assay
rMZ-II and recombinant prothrombin were activated by prothrombinase prior to the experiment using conditions previously described (49). Subsequent gel electrophoresis analyses under reducing conditions were performed to verify that both rMZ-II and the recombinant prothrombin preparations were activated to the same extent and that rMZ-IIa and recombinant α-thrombin formed had comparable amounts of B chain. The enzymes (rMZ-IIa and recombinant α-thrombin) used for the titration of Spetrozyme-TH and S-2238 were assayed at a constant concentration (4.3 nM), as previously described using serial dilutions of chromogenic substrate (45). The absorbance was monitored with a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA). The data were plotted according to the Michaelis−Menten equation using Prizm (Graphpad Software Inc., San Diego, CA). A direct comparison between active recombinant meizothrombin (rMZ-IIa) and recombinant α-thrombin, for cleavage of spetrozyme-TH and S-2238, demonstrated that the Vmax values of rMZ-IIa for Spectrozyme-TH and S-2238 are ∼2- and 1.5-fold higher, respectively, than the Vmax of recombinant α-thrombin for the same substrates. These data confirm the findings in the literature demonstrating the fact that meizothrombin has higher catalytic efficiency than α-thrombin toward the chromogenic peptidyl substrates generally used to assess for α-thrombin activity (29–31,38).
Functionally defined apparent dissociation constants (KDapp) for factor Va binding to factor Xa-PCPS were obtained from plots measuring the rate of α-thrombin generation as a function of factor Va concentration in the presence of a limiting (constant) concentration of factor Xa. Throughout all experiments, the assumption was n = moles of factor Xa bound per mole of factor Va at saturation; throughout this study n = 1. The stoichiometry of the factor Va−factor Xa interaction was fixed at 1. The initial rate of formation of α-thrombin (initial velocity in nM IIa min−1) was calculated, and the data were analyzed and plotted using Prizm according to the one-binding site model. Dissociation constants were extracted directly from the graphs.
The assay using purified reagents and verifying the activity of the recombinant factor V molecules was conducted under conditions where all factor Xa was saturated with factor Va, as described by assessing α-thrombin formation by the change in the absorbance of a chromogenic substrate at 405 nm (Spectrozyme-TH, 0.4 mM) (43,45). All factor V molecules were activated with RVV-V activator or α-thrombin as described previously (21,35). Knowing the KDapp of each factor Va species for factor Xa, we calculated the amount necessary to saturate factor Xa using the quadratic equation described in the literature (50) before each experiment. The total concentration of ligand (VaT) used was modified appropriately to yield 95−98% saturation of the factor Xa molecule. The absorbance was monitored with a Thermomax microplate reader and compared to that of a α-thrombin standard prepared daily using purified plasma-derived α-thrombin. The data were analyzed and plotted using Prizm according to the Michaelis−Menten equation. Kinetic constants provided throughout the paper were extracted directly from the graphs.
Results
Activation of Recombinant Human Factor V Molecules
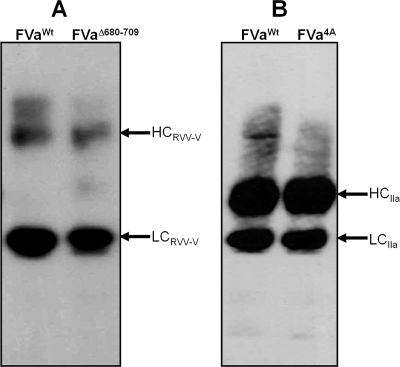
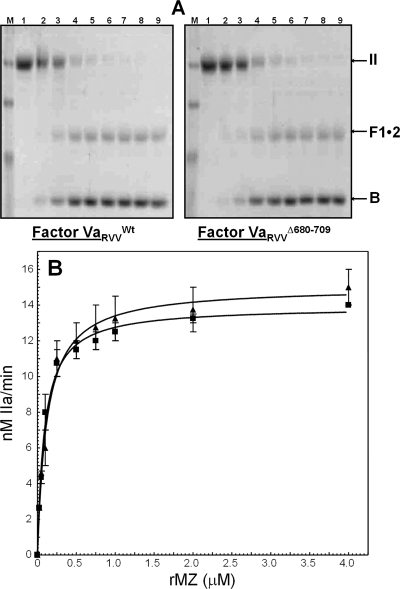
To ascertain the function of the acidic region from the COOH terminus of factor Va heavy chain for cofactor activity, we constructed a molecule that is missing the entire amino acid region 680−709 [factor VΔ680−709 (Figure 1)]. We have also constructed a factor V molecule with the 695DYDY698 → AAAA mutations (factor V4A). The recombinant molecules were expressed in mammalian cells and purified to homogeneity as described previously (44). Since factor VΔ680−709 is missing Arg709 that is the activating cleavage site for α-thrombin (Figure 1), in the functional assays, the recombinant mutant molecule was activated with RVV-V activator. In contrast, factor V4A was activated with either RVV-V activator or α-thrombin. SDS−PAGE analyses followed by immunoblotting with specific monoclonal antibodies to the heavy and light chain of the cofactor demonstrate that the mutant recombinant proteins were intact, homogeneous, and migrated according to their expected molecular weights (Figure 2). In addition, control experiments using a polyclonal antibody to factor V demonstrate that all factor V molecules were fully activated prior to use.
Figure 2.
Electrophoretic analyses of wild-type factor V and recombinant factor V molecules. (A) Factor VWt and factor VΔ680−709 were activated with RVV-V activator. (B) Factor VWt and factor V4A were activated with α-thrombin as described in and analyzed by SDS−PAGE. After being transferred to a PVDF membrane, immunoreactive fragments were detected with monoclonal antibodies αHFVaHC17 (recognizing an epitope on the heavy chain of the cofactor between amino acid residues 307 and 506) and αHFVaHC9 (recognizing the light chain). At the right, the positions of the heavy and light chains of factor Va are shown.
Cofactor Function of Recombinant Human Factor Va Molecules
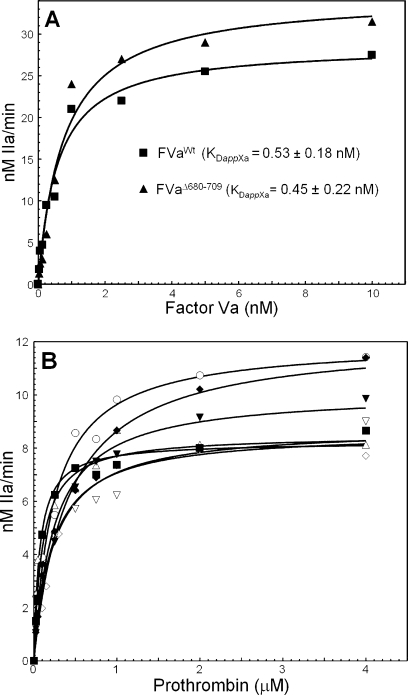
Recent work using recombinant factor Va molecules demonstrated that all cofactors lacking portions or the entire acidic COOH terminus of the heavy chain encompassing amino acid region 680−709 had KDapp values for membrane-bound factor Xa that are similar to the values for the bimolecular interaction found with the wild type or the intact plasma cofactor (37). These values are similar to the KDapp obtained with truncated plasma-derived factor Va molecules for factor Xa (20,21). Our studies with factor VaΔ680−709 also demonstrate that the mutant cofactor has an affinity for plasma-derived factor Xa similar to that of the wild-type molecule (Figure 3A). Similar results were found for factor Va4A (not shown). Thus, elimination of the acidic COOH-terminal region of the factor Va heavy chain (amino acid residues 680−709) is of no consequence with respect to the affinity of the cofactor for factor Xa.
Figure 3.
Raw data used for the determination of the parameters of prothrombinase complex assembly and function. (A) Determination of the affinity of recombinant factor Va molecules for plasma-derived factor Xa. Initial rates of α-thrombin generation were determined as described in . Data for prothrombinase assembled with factor VaWt are shown with filled squares (R2 = 0.979), while data for prothrombinase assembled with FVaΔ680−709 are depicted with filled triangles (R2 = 0.978). Titrations were carried out to 20 nM factor Va; however, for graphical purposes, the data show the titration up to 10 nM cofactor. The solid lines represent a nonlinear regression fit of the data as detailed in using Prizm and the model for one binding site. The apparent dissociation constant (KDapp) for each species was obtained from each titration performed at least in triplicate with at least two different preparations of recombinant proteins and is listed in the inset. (B) Determination of kinetic parameters of prothrombinase assembled with various factor Va species. Initial rates of α-thrombin generation were determined using the KD for factor Xa found in panel A to determine the concentration of factor Va as described in in the presence of PCPS vesicles and factor Xa. Data for prothrombinase assembled with two different concentrations of factor VaWtRVV are shown with empty triangles (10 nM, 95% factor Xa saturation; R2 = 0.97) and empty inverted triangles (20 nM, 97% factor Xa saturation; R2 = 0.97). Data for prothrombinase assembled with two different concentrations of factor VaΔ680−709 are depicted with filled diamonds (10 nM, 96% factor Xa saturation; R2 = 0.98) and empty circles (20 nM, 98% factor Xa saturation; R2 = 0.99), while data for prothrombinase assembled with factor VaIIaPLASMA are depicted with filled squares (10 nM, 98% factor Xa saturation; R2 = 0.97) and with factor VaRVVPLASMA with empty diamonds (10 nM, 98% factor Xa saturation; R2 = 0.97). Data for prothrombinase assembled with factor VaRVV4A are depicted with filled inverted triangles (10 nM, 96.5% factor Xa saturation; R2 = 0.98). The values of Km and Vmax/ET (= kcat) extracted directly from these graphs are listed in Table 1.
Several studies have shown that plasma-derived or recombinant meizothrombin has increased activity (kcat) toward chromogenic or fluorescent substrates used to assess α-thrombin activity (8,25,31,32,38). This fact had been established previously for DAPA (8,32) and TAME (30). One early study comparing the amidolytic activities between α-thrombin and meizothrombin using S-2238 demonstrated an ∼25% increase in the kcat and an ∼18% increase in the second-order rate constant of meizothrombin for S-2238 as compared to the kcat and the second-order rate constant of α-thrombin for the same substrate (31), and the general consensus is that there are subtle but significant differences between the active sites of α-thrombin and meizothrombin (32,33). We evaluated the ability of all recombinant factor Va molecules to function as a cofactor for prothrombinase. A clotting assay using factor V-deficient plasma and an α-thrombin generation assay using Spectrozyme-TH and purified reagents were employed. In addition, we have followed the activation of prothrombin by gel electrophoresis. Figure 3B illustrates the fitted hyperbolic plots used to extract the kinetic constants obtained following plasma-derived prothrombin activation by prothrombinase assembled with all control or modified cofactor molecules, and summarized in Table 1. Because we wanted to prevent the possibility that differences between prothrombinase assembled with factor VaWt and prothrombinase assembled with factor VaΔ680−709 or factor Va4A may be attributed to subtle differences in the KDapp of factor Va for factor Xa, which would result in a smaller amount of prothrombinase formed, all experiments described below were conducted under conditions in which more than 95% of factor Xa was saturated with factor Va. The amount of factor Xa saturation by each cofactor was calculated using the quadratic equation provided in the literature (50) and the KDapp obtained from the data shown in Figure 3A. The percent saturation of factor Xa by each cofactor species and the correlation coefficient (R2) of the fitted hyperbolic plots used to extract the values of Vmax and Km are provided in the legend of Figure 3B together with the actual reagent concentrations used in each experiment.
Table 1. Characteristics of Various Factor Va Molecules Assembled into Prothrombinasea.
| FVaIIaPLASMA | FVaRVVPLASMA | FVaIIaWt | FVaRVVWt | FVaRVVΔ680−709 | FVaIIa/CGd | FVaIIa/NN | FVaIIa4A | FVaRVV4A | |
|---|---|---|---|---|---|---|---|---|---|
| II Consumptionb[mol s−1 (mol of fXa)−1] | 16.2 ± 1.8 | NDf | 17.3 ± 1.2 | 15.8 ± 1.1 | 5.1 ± 0.54 | 5.9 ± 0.6 | NDf | 4.4 ± 0.42 | 4.7 ± 0.4 |
| Km (μM) | 0.1 ± 0.011 | 0.24 ± 0.04 | 0.1 ± 0.013 | 0.22 ± 0.04 | 0.24 ± 0.03 | 0.26 ± 0.03 | 0.15 ± 0.07 | 0.31 ± 0.1 | 0.25 ± 0.03 |
| kcat (min−1) | 1715 ± 45 | 1754 ± 84 | 1697 ± 48 | 1721 ± 90 | 2396 ± 71 | 2122 ± 62 | 2066 ± 86 | 2031 ± 81 | 2018 ± 75 |
| specific activity (units/mg)c | 3337 ± 480 | 2753 ± 267 | 2926 ± 340 | 3200 ± 550 | 869 ± 192 | 1280 ± 114 | NDe,f | 663 ± 210 | 640 ± 120 |
The rate of thrombin formation following activation of prothrombin by prothrombinase assembled with the various factor Va species was calculated as described in with the knowledge of the dissociation constant of each factor Va species for factor Xa. In each case, more than 95% of factor Xa was saturated with factor Va. Some of the data in the table were extracted directly from the graphs shown in Figure 3B.
The rate of prothrombin consumption was determined following quantitative scanning densitometry of several gels stained with Coomassie Blue as described in . Some of the gels used are shown in Figure 4.
All clotting activities were determined in a two-stage clotting assay following activation of factor V species by RVV-V activator or α-thrombin as described previously (39,40).
Plasma factor V was activated with α-thrombin and treated with cathepsin G (CG) as described previously (20).
The clotting activity of the truncated cofactor is ∼50% of that of plasma-derived factor Va (21).
Not determined in this study.
Factor VaRVVΔ680−709 had a 39.2% greater kcat [Figure 3B (○)] and 73% lower clotting activity than factor VaRVVWt [Figure 3B (▽)], while factor VaIIa/CG had a 23.7% greater kcat and 62% lower clotting activity than factor VaIIaPLASMA (Table 1). In separate experiments, we have found that under similar experimental conditions, factor VaIIa/NN that has a 50% lower clotting activity than the plasma cofactor (21) had a 20.5% increased kcat compared to that of factor VaIIaPLASMA (Table 1). All these data are in complete agreement with earlier and recent findings (18–21,37) and demonstrate that elimination of the COOH-terminal region of the factor Va heavy chain produces a molecule with poor clotting activity, yet when the truncated cofactor is incorporated into prothrombinase, it produces an enzyme with increased catalytic efficiency for prothrombin activation as assessed using purified components and under conditions in which the majority of membrane-bound cofactor is bound to factor Xa. Overall, the data listed in Table 1 also demonstrate that the plasma and wild-type molecule behave similarly following activation by either RVV-V activator or α-thrombin with respect to prothrombin activation. These data are in complete accord with earlier findings (20,21) and demonstrate that plasma and wild-type factor Va are interchangeable. As a consequence, throughout this work control experiments were conducted, and their results are shown with only one of the two molecules as indicated.
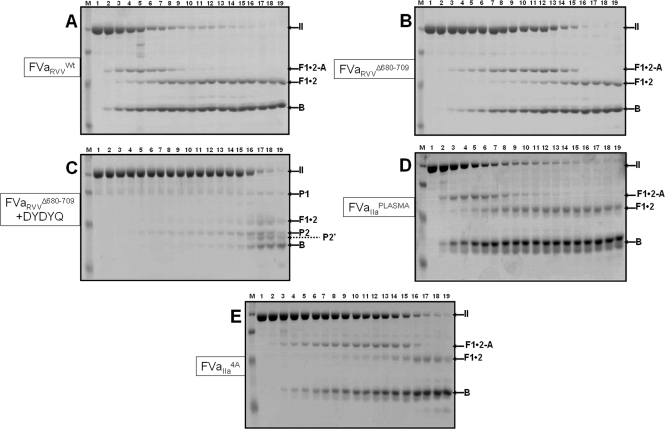
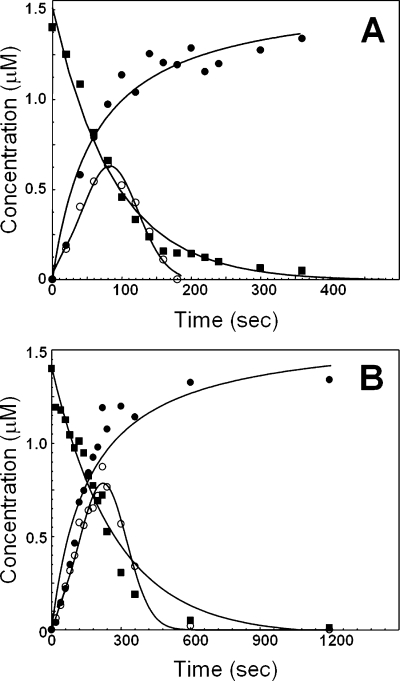
We next evaluated the ability of prothrombinase assembled with the truncated molecules to activate prothrombin by gel electrophoresis. Under the conditions that were employed, prothrombinase assembled with factor VaRVVWt activates prothrombin quickly, with meizothrombin as a short-lived intermediate as demonstrated by the brief half-life of fragment 1·2-A (Figure 4A). Scanning densitometry demonstrated a peak of meizothrombin early in the reaction at 80 s, and no meizothrombin was detected following 180 s (Figure 5A). In contrast, in the presence of prothrombinase assembled with factor VaΔ680−709, prothrombin is consumed with a rate that is approximately 3-fold slower than the rate of consumption of prothrombin by prothrombinase assembled with the wild-type molecule (Table 1), with persistence of meizothrombin as indicated by the lingering of fragment 1·2-A even at the late time points of the reaction (Figure 4B). Scanning densitometry demonstrated a peak of meizothrombin late in the reaction at 250 s. Meizothrombin was more concentrated and persisted for up to 6 min (Figure 5B). In addition, the rate of appearance of the B chain is also delayed (by approximately 3-fold) when prothrombin is activated by prothrombinase assembled with factor VaΔ680−709, compared with the rate of appearance of the B chain obtained following incubation of prothrombin with prothrombinase assembled with factor VaRVVWt. Similar results were obtained with factor VaIIa/CG (Table 1). In contrast, in the presence of DYDYQ, no meizothrombin is observed following prothrombin activation by prothrombinase assembled with factor VaΔ680−709, and α-thrombin is formed through the alternate pathway characterized by initial cleavage at Arg271 and formation of prethrombin 2 as the intermediate (Figure 4C). Overall, the data demonstrate that elimination of amino acid region 680−709 from factor Va results in a cofactor molecule that when incorporated into prothrombinase produces an enzyme responsible for both delayed prothrombin activation and persistence of meizothrombin during activation of prothrombin.
Figure 4.
Analysis of the activation of plasma-derived prothrombin by prothrombinase. Plasma-derived prothrombin (1.4 μM) was incubated in different mixtures with PCPS vesicles (20 μM), and prothrombinase assembled with either wild-type factor Va [(A) 10 nM] or factor VaΔ680−709 [(B) 10 nM] as described in , and the reaction was started by the addition of factor Xa. (C) Prothrombinase assembled with factor VaΔ680−709 in the presence of 20 μM DYDYQ (same conditions as in panel B). (D) Prothrombinase assembled with plasma-derived factor Va (10 nM). (E) Prothrombinase assembled with factor Va4A (10 nM). At selected time intervals, aliquots of the reaction mixtures were withdrawn and treated as described in . Lane M contained the molecular weight markers (from top to bottom): 98000, 64000, 50000, 36000, and 22000. Lanes 1−19 represent samples from the reaction mixture before (0 min) the addition of factor Xa and 20 s, 40 s, 60 s, 80 s, 100 s, 120 s, 140 s, 160 s, 180 s, 200 s, 220 s, 240 s, 5 min, 6 min, 10 min, 20 min, 30 min, and 60 min, respectively, following the addition of factor Xa. The prothrombin-derived fragments are shown as follows: II, prothrombin (amino acid residues 1−579); prethrombin-1 (amino acid residues 156−579); F1·2-A, fragment 1·2-A chain (amino acid residues 1−320); F1·2, fragment 1·2 (amino acid residues 1−271); P2, prethrombin-2 (amino acid residues 272−579); P2′, prethrombin-2 cleaved at Arg284; B, B chain of α-thrombin (amino acid residues 321−579).
Figure 5.
Reaction profiles for the activation of prothrombin by prothrombinase. Progress curves for products and reactants for the activation of prothrombin by prothrombinase assembled with factor VaWt (A) or factor VaΔ680−709 (B) were obtained by quantitative scanning densitometry of gels shown in panels A and B of Figure 4, as described in . The graphs illustrate the disappearance of prothrombin (◼), the transient formation of meizothrombin (○), and the accumulation of the B chain of α-thrombin (●). There is a 3-fold difference in the x-axis between the two panels, because prothrombinase assembled with factor VaΔ680−709 consumes prothrombin with an ∼3-fold slower rate than prothrombinase assembled with factor VaWt. The lines for the disappearance of prothrombin were drawn according to the equation of a one-phase exponential decay (for factor VaWt, R2 = 0.985; and for factor VaΔ680−709, R2 = 0.968). The lines depicting the formation of meizothrombin and the accumulation of the B chain of α-thrombin were arbitrarily drawn. Additional data points extending to incubation for 1 h (shown in Figure 4) have been omitted for clarity.
In preliminary experiments using several preparations of recombinant proteins, we observed that while the truncated factor Va molecules are impaired in their clotting activity, factor Va4A is also deficient in its clotting activity. To understand the properties of this molecule and the effect of the mutations on cofactor activity, we used the same preparation of recombinant protein to perform three different experiments. Factor V4A was first activated with α-thrombin, and the solution was split into three separate samples. One sample was used for assessment of clotting activity, one sample was used to measure the kinetic parameters of prothrombinase assembled with a saturating concentration of factor VaIIa4A, and the third sample was used for analysis of prothrombin activation by gel electrophoresis. The results reveal that while factor VaIIa4A is severely impaired in its clotting activity (∼22% of that of factor VaIIaWt), prothrombinase assembled with the mutant molecule shows a 19.7% increased kcat (Table 1). Gel electrophoresis followed by scanning densitometry analysis demonstrated that prothrombinase assembled with factor VaIIa4A cleaves prothrombin with a rate that is approximately 3.8-fold slower than the rate of cleavage of prothrombin assembled with factor VaIIaWt or factor VaIIaPLASMA (Figure 4D,E and Table 1). Scanning densitometry of several gels studying prothrombin activation by prothrombinase assembled with factor VaIIa4A revealed a peak of meizothrombin at approximately 240 s with significant amounts of meizothrombin remaining for up to 10 min (not shown). The data also showed more meizothrombin formed and delayed B chain formation by prothrombinase assembled with factor Va4A as compared to the levels of meizothrombin produced by prothrombinase assembled with factor VaWt. Similar data were obtained with factor VaRVV4A (Table 1). These findings strongly support our previous conclusion suggesting that amino acid sequence 695−699 regulates meizothrombin formation by prothrombinase (35,36). These data also demonstrate that, in the presence of prothrombinase assembled with factor Va4A, the excess meizothrombin formed as assessed functionally by clotting assays can compensate for the absence of α-thrombin in the assay using purified reagents because the increased amidolytic activity of meizothrombin toward the chromogenic substrate is read as α-thrombin activity.
Role of the Acidic COOH-Terminal Portion of the Factor Va Heavy Chain: A Potential Mechanism
The data obtained thus far studying plasma-derived prothrombin activation by prothrombinase assembled with the mutant cofactor molecules suggest that meizothrombin formation is not greatly delayed but rather that meizothrombin accumulates to higher and more persistent levels and lingers throughout the time course (Figure 5B). The appearance of the B chain of α-thrombin is also delayed when prothrombin is activated by prothrombinase assembled with the mutant cofactor (Figures 4 and 5). These data indicate that one or both of the two prothrombin activation cleavages are impaired (affected) when prothrombinase is assembled with a cofactor molecule missing the acidic COOH terminus of the heavy chain or with factor Va4A. To verify if under the conditions employed, cleavage at Arg320 in prothrombin is delayed when prothrombinase is assembled with factor VaIIa4A as compared to cleavage of prothrombin at the same site by prothrombinase assembled with factor VaIIaWt, we studied prothrombin cleavage during the first 90 s of the reaction. Quantitative scanning densitometry of the gels revealed no differences in the rate of plasma-derived prothrombin consumption by prothrombinase assembled with either factor VaIIa4A or factor VaWt during this period of time (not shown). These data combined with the data shown in Figures 4 and 5 illustrating delayed prothrombin consumption which is accompanied by an increase in the concentration of meizothrombin (up to ∼20%) in the case of the activation of prothrombin by prothrombinase assembled with factor VaΔ680−709 as compared to the concentration of meizothrombin produced following activation of prothrombin by prothrombinase assembled with factor VaWt suggest that delayed initial cleavage at Arg320 in plasma-derived prothrombin is not a direct consequence of the mutation in factor Va, but rather the result of product inhibition by the accumulating meizothrombin. This inhibition is observed only late in the reaction when sufficient meizothrombin accumulates, as clearly demonstrated by our combined results.
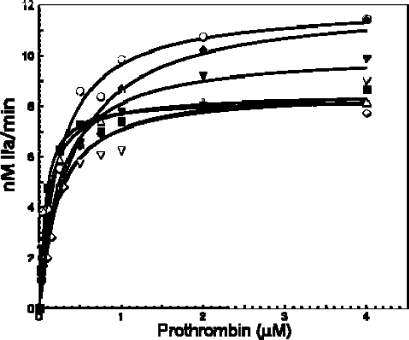
It is important to note that during the course of the assay using plasma-derived prothrombin we are measuring the sum of meizothrombin and α-thrombin. A more rigorous test to verify which cleavage in prothrombin is affected by the deletion at the COOH terminus of factor Va heavy chain involves the study of the activation of a prothrombin molecule that cannot be cleaved at Arg271 such as rMZ-II (41). In addition, while cleavage of rMZ-II can be followed by gel electrophoresis, generation of rMZ-IIa can be also assessed with the chromogenic substrate used to assess α-thrombin formation. We thus followed activation of rMZ-II by prothrombinase assembled with either factor VaRVVΔ680−709 or factor VaRVVWt by gel electrophoresis (Figure 6A) and activity assays using Spectrozyme-TH (Figure 6B). Both sets of data demonstrate that there are no significant differences between the rates of activation of rMZ-II by either enzyme. The rates of rMZ-II consumption were similar as determined by scanning densitometry of the gels depicted in Figure 6A, and the profiles of both titrations are coincident [similar Vmax values and similar Km values of 91 ± 9 and 121 ± 18 μM (Figure 6B)]. Comparable results were found in three independent measurements using two different preparations of recombinant factor Va molecules and two separate preparations of rMZ-II. The data confirm our overall findings with plasma-derived prothrombin and demonstrate that prothrombinase-mediated cleavage at Arg320 in prothrombin is not affected by the COOH-terminal portion of the factor Va heavy chain.
Figure 6.
Analysis of the activation of rMZ-II. (A) Gel electrophoretic analyses. rMZ-II (1.4 μM) was incubated in different mixtures with PCPS vesicles (20 μM), DAPA (3 μM), and factor VaWt (left panel, 20 nM) or factor VaΔ680−709 (right panel, 20 nM). The reaction was started by the addition of factor Xa, and the samples were treated as detailed in . Lanes 1−9 contained samples of the reaction mixture following incubation of prothrombinase with rMZ-II before (lane 1) or following incubation for 0.5, 1, 2.5, 4, 6, 10, 20, and 30 min with factor Xa, respectively. Positions of prothrombin-derived fragments are indicated at the right as detailed in the legend of Figure 4. The factor Va species used for the reconstitution of prothrombinase are shown under each panel. (B) Kinetic analyses. Initial rates of α-thrombin generation were determined as described in , and the data were plotted according to the Michaelis−Menten equation. Data for activation of rMZ-II by prothrombinase assembled with factor VaWt are shown with filled squares (R2 = 0.98), while data for activation of rMZ-II by prothrombinase assembled with factor VaΔ680−709 are depicted with filled triangles (R2 = 0.96). Kinetic constants reported in the text were extracted directly from the graphs.
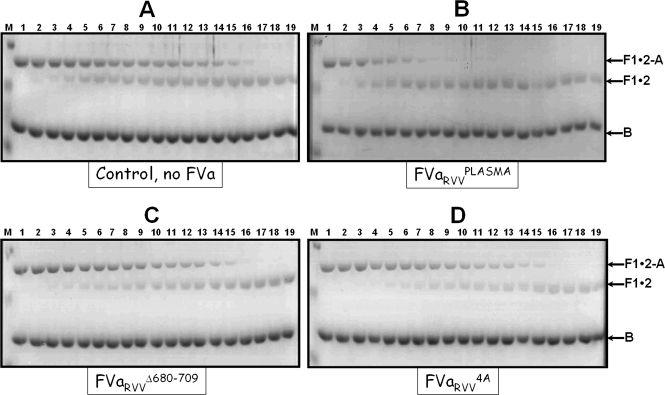
Analysis of the results obtained thus far suggests that cleavage of meizothrombin at Arg271 by prothrombinase assembled with factor VaRVVΔ680−709 may be slower than cleavage of meizothrombin at the same site by prothrombinase assembled with factor VaRVVWt, resulting in slower conversion of meizothrombin to α-thrombin. We next compared the rate of cleavage of FPR-meizothrombin by prothrombinase assembled with various factor Va molecules (Figure 7). The data demonstrate a delay for cleavage of FPR-meizothrombin at Arg271 by prothrombinase assembled with factor VaRVVΔ680−709 (panel C) or factor VaRVV4A (panel D) as compared to the same reaction catalyzed by prothrombinase assembled with factor VaRVVPLASMA (panel B) or factor VaRVVWt (not shown). A direct comparison between the rates of cleavage of FPR-meizothrombin by membrane-bound factor Xa alone (Figure 7A) or by prothrombinase assembled with either factor VaRVVΔ680−709 (Figure 7C) or factor VaRVV4A (Figure 7D) does not show any significant differences. These data imply that following incorporation of factor Va into prothrombinase it is the acidic COOH-terminal hirudin-like portion of the factor Va heavy chain and in particular amino acid sequence 695DYDY698 that is responsible for the increase in the rate of cleavage of meizothrombin at Arg271. Quantitative scanning densitometry of fragment 1·2-A present on the gels shown in Figure 7 demonstrated an ∼3−5-fold delay in cleavage of FPR-meizothrombin at Arg271 by either membrane-bound factor Xa alone or prothrombinase assembled with the recombinant mutant factor Va molecules, compared to cleavage at Arg271 by prothrombinase assembled with the plasma-derived cofactor (Figure 7B) or factor VaWt. These data are in complete agreement with all the findings presented herein and demonstrate that the lingering of meizothrombin during plasma-derived prothrombin activation by prothrombinase assembled with factor VaRVVΔ680−709 or factor Va4A is the result of impaired cleavage of the molecule at Arg271. Overall, the findings suggest that amino acid sequence 695DYDY698 from the COOH-terminal portion of the factor Va heavy chain regulates meizothrombin concentration (processing) during activation of prothrombin by prothrombinase.
Figure 7.
Gel electrophoretic analyses for cleavage of FPR-meizothrombin. FPR-meizothrombin (1.4 μM) was incubated in different mixtures with PCPS vesicles (20 μM) and factor Va as described in the legend of Figure 4. The reaction and the samples were further treated, scanned, and quantified as detailed in : (A) control, no factor Va [13.4 mol of FPR-meizo consumed s−1 (mol of factor Xa)−1], (B) factor VaRVVPLASMA [42.6 mol of FPR-meizo consumed s−1 (mol of factor Xa)−1], (C) factor VaRVVΔ680−709 [8.4 mol of FPR-meizo consumed s−1 (mol of factor Xa)−1], and (D) factor VaRVV4A [14.6 mol of FPR-meizo consumed s−1 (mol of factor Xa)−1]. Lane M contained the molecular weight markers (from top to bottom): 50000, 36000, and 22000. Lanes 1−19 contained samples from the reaction mixture before (0 min) the addition of factor Xa and 20 s, 40 s, 60 s, 80 s, 100 s, 120 s, 140 s, 160 s, 180 s, 200 s, 220 s, 240 s, 5 min, 6 min, 10 min, 20 min, 30 min, and 60 min, respectively, following the addition of factor Xa. The prothrombin-derived fragments are shown as detailed in the legend of Figure 4. The factor Va species used for the reconstitution of prothrombinase are also shown under each panel.
Discussion
Our data demonstrate that the acidic COOH terminus of the factor Va heavy chain is responsible for coordinate activation of prothrombin, resulting in timely α-thrombin formation at the place of vascular injury. Furthermore, our findings appear to resolve many apparently conflicting results and explain the observation that was initially reported 15 years ago (18) and verified several times with either truncated plasma-derived factor Va (19–21) or more recently with recombinant factor Va molecules (37). Namely, incorporation of cofactor molecules with a truncated heavy chain into prothrombinase results in the enhanced stability of meizothrombin during prothrombin activation. Our findings assign an important physiological role to the COOH terminus of the factor Va heavy chain for efficient prothrombin activation and confirm the fact that the sequence 695DYDY698 regulates the activity of factor Xa within prothrombinase (36).
We show that while rMZ-II is cleaved with equivalent rates by either prothrombinase assembled with factor VaWt or prothrombinase made with factor VaΔ680−709, acceleration of the rate of FPR-meizothrombin cleavage at Arg271 by prothrombinase assembled with factor Va4A or factor VaΔ680−709 is impaired, compared to the rate of cleavage at the same bond by prothrombinase assembled with factor VaPLASMA or factor VaWt. Altogether, these findings demonstrate that while the rate of the first cleavage in prothrombin (i.e., Arg320) is not significantly affected by the deletion of the COOH-terminal portion of the factor Va heavy chain, the rate of cleavage of meizothrombin at Arg271 by prothrombinase appears to be regulated by the acidic hirudin-like COOH-terminal portion of the heavy chain. Our data show that prothrombinase assembled with factor VaPLASMA or factor VaWt cleaves FPR-meizothrombin with a rate that is 4−5-fold higher than the rate of cleavage of FPR-meizothrombin by factor Xa alone or by prothrombinase assembled with the mutant molecules. These findings are in complete accord with earlier data demonstrating that incorporation of factor Va into prothrombinase results in a 3−5-fold increase in the rate of cleavage of meizothrombin at Arg271 as compared to the rate of cleavage at the same site by factor Xa alone (7,8).
Recently, we have demonstrated that formation of meizothrombin by prothrombinase assembled with the plasma-derived cofactor is inhibited in the presence of low concentrations of an acidic region from the carboxyl-terminal portion of factor Va (represented by pentapeptide DYDYQ) (36). The combined findings demonstrate that amino acid sequence 695−698 is responsible for the regulation of meizothrombin processing and suggest that peptide DYDYQ inhibits meizothrombin generation by prothrombinase by accelerating the rate of cleavage at Arg271 by the enzyme, thus practically alleviating the factor Va cofactor effect within prothrombinase. As a result, in the presence of DYDYQ, cleavage of prothrombin by prothrombinase becomes virtually independent of factor Va. These findings are in complete accord with our recent data demonstrating that DYDYQ significantly increases the rate of cleavage of prothrombin consumption by membrane-bound factor Xa alone because of acceleration of the cleavage at Arg271(36). Indeed, membrane-bound factor Xa/DYDYQ, in the absence of factor Va, cleaves prothrombin with a rate that is ∼5-fold slower than the rate of cleavage of prothrombin by prothrombinase (ref (36) and Table 1), while membrane-bound factor Xa alone cleaves prothrombin with a rate that is ∼250-fold slower than the rate of cleavage of the substrate by prothrombinase. However, it is noteworthy that in the presence of an excess of DYDYQ, prothrombin cleavage by prothrombinase is significantly inhibited (36). Altogether, these findings warrant the need for further investigation of the mode of action and site of interaction with prothrombin of the pentapeptide as well as of the molecular mechanism of inhibition of prothrombinase by DYDYQ.
Blood clotting enzymes, with the exception of factor IX and prothrombin, are activated following single proteolytic cleavage (1). It is thus assumed that results obtained following the activation of a zymogen either by gel electrophoresis or by assays that measure the generation of enzymatic activity with chromogenic substrates should be concordant. However, prothrombin activation is different from the activation of other zymogens because (1) prothrombin can be activated through two different pathways that have different requirements and significantly different rates and (2) activation of prothrombin through the meizothrombin pathway generates an intermediate that has higher amidolytic activity than α-thrombin toward chromogenic substrates that are commonly used to assess α-thrombin generation. Thus, while following prothrombin activation by both gel electrophoresis and activity assays simultaneously may appear to be a duplication of the same result, drawing conclusions from activity assays alone without analyzing the pathway to prothrombin activation and the intermediates formed is an oversimplification that can lead to erroneous conclusions.
Two assays are used worldwide for the determination of factor Va cofactor activity: a clotting assay using factor V-deficient plasma and a prothrombinase assay using purified reagents. The former assay is performed at low (limiting) concentrations of factor Va, while the latter assay is conducted with high (saturating) concentrations of factor Va with respect to factor Xa. Both assays indirectly report on factor Va cofactor activity through the activation of prothrombin to α-thrombin. The clotting time measures the level of fibrin formation in factor V-deficient plasma, while the prothrombinase assay measures α-thrombin’s amidolytic activity as it is generated toward a chromogenic substrate. Initial cleavage of prothrombin at Arg320 by prothrombinase, which is absolutely factor Va-dependent, results in rapid meizothrombin generation. However, depending on the ratio of meizothrombin to α-thrombin formed at the moment the measurement is taken, the results reflect the clotting or amidolytic activity of either α-thrombin or meizothrombin or the sum of both. Thus, if generation of α-thrombin is delayed compared to the formation of meizothrombin, the measurements will mostly reflect the properties of meizothrombin (i.e., impaired clotting and increased chromogenic activity).
Work performed independently in several laboratories worldwide has demonstrated that while elimination of the COOH-terminal acidic region of factor Va heavy chain results in a cofactor molecule with poor clotting activity (retaining 20−40% of the clotting activity of normal factor Va), the same molecule as part of prothrombinase produces a substantial increase in the kcat of the enzyme in an assay using a chromogenic substrate to assess α-thrombin generation (18–21) (Table 2). Similar results were recently obtained with recombinant factor Va molecules missing portions of the COOH terminus of the factor Va heavy chain (37). All these results could be partially explained if the truncated cofactor molecules had weakened binding capabilities for membrane-bound factor Xa. However, previously reported data demonstrated that all truncated factor Va molecules have KD values for factor Xa similar to that of the plasma-derived cofactor (20,21), and recent experiments with recombinant factor Va molecules missing the entire hirudin-like COOH-terminal portion of the factor Va heavy chain (amino acids 679−709, rFVa678) confirm our findings shown in Figure 3A with factor VaΔ680−709 and demonstrate that the recombinant mutant cofactor binds factor Xa with an affinity similar to that of the wild-type molecule (37). Thus, while every study was remarkably consistent in showing poor clotting and increased kcat for the same truncated factor Va molecules [up to ∼50% higher kcat(37) (Table 2)], no satisfactory explanation has been yet provided to explain this seeming paradox at best (18–21), or at worst the observations were simply discarded as relatively modest changes (37). Our data, put in the context of the literature, provide a logical explanation for these observations and demonstrate that the acidic COOH-terminal portion of the factor Va heavy chain controls the rate of α-thrombin generation by factor Xa within prothrombinase by controlling the rate of meizothrombin cleavage at Arg271. Our findings are thus consistent with the interpretation that amino acid sequence 695DYDY698 of the heavy chain of the cofactor is a significant contributor to the macromolecular substrate recognition of prothrombin by prothrombinase.
Table 2. Summary of Results Obtained with Factor Va Molecules Truncated at the COOH Terminus of the Heavy Chain in a Clotting Assay and in a Prothrombinase Assay.
| impaired clottinga | increased kcatb | |
|---|---|---|
| Gerads et al. (18) | + | + (17%)c |
| Bakker et al. (19) | +d | + (12%)e |
| Camire et al. (20) | + | + (18%) |
| Kalafatis et al. (21) | + | + (19%)f |
| Toso and Camire (37) | + | + (29−50%)g |
The plus sign indicates that the molecules missing part or the entire COOH-terminal portion of the heavy chain are impaired in clotting activity.
The plus sign indicates that the factor Va molecules missing part or the entire COOH-terminal portion of the heavy chain have increased catalytic efficiency when they are assembled in prothrombinase. In parentheses are the percent increases in kcat compared to that of prothrombinase assembled with plasma-derived factor Va or to that of wild-type recombinant factor Va reported in each study.
All results reported were obtained with proteins of bovine origin.
From ref (18); the authors also report results obtained with human factor Va and clotting assays with factor V-deficient plasma.
All work was performed with proteins of human origin and is complementary to the work reported in ref (18).
The kcat of factor VaIIa/NN in the assay using purified reagents and a chromogenic substrate to assess for α-thrombin formation is reported here (Table 1).
All results were obtained with recombinant proteins. The factor V construct used for expression was missing a major portion of the B domain of the molecule.
Acknowledgments
We thank Dr. Ken Mann and Dr. Tom Orfeo from the Department of Biochemistry of the University of Vermont for providing monoclonal antibodies to factor V and the purified enzyme from Naja nigricollis nigricollis and Dr. Michael Nesheim and Paul Kim from the Department of Biochemistry at Queen’s University (Kingston, ON) for the recombinant prothrombin molecules. Finally, we thank Dr. Susan Kennedy-Kalafatis and Dr. Edward Plow for helpful advice and for critical reading of the manuscript.
Funding Statement
National Institutes of Health, United States
Footnotes
Abbreviations: PS, l-α-phosphatidylserine; PC, l-α-phosphatidylcholine; PCPS vesicles, small unilamellar phospholipid vesicles composed of 75% PC and 25% PS (w/w); SDS−PAGE, sodium dodecyl sulfate−polyacrylamide gel electrophoresis; PVDF, polyvinylidene difluoride; ABE-I, anion binding exosite I; ABE-II, anion binding exosite II; DYDYQ, pentapeptide from factor Va heavy chain with the sequence Asp695-Tyr696-Asp697-Tyr698-Gln699; factor VWt, wild-type recombinant human factor V; factor VaIIaWt, wild-type recombinant human factor V activated with α-thrombin; factor VaRVVWt, wild-type recombinant human factor V activated with RVV-V activator; factor VΔ680−709, recombinant human factor V missing amino acid residues 680−709; factor VaRVVΔ680−709, recombinant human factor V missing amino acid residues 680−709 activated with RVV-V activator; factor V4A, recombinant human factor V with the 695DYDY698 → AAAA mutations; factor VaIIa4A, recombinant human factor V with the 695DYDY698 → AAAA mutations activated with α-thrombin; factor VaRVV4A, recombinant human factor V with the 695DYDY698 → AAAA mutations activated with RVV-V activator; factor VIIaPLASMA, plasma-derived human factor V activated with α-thrombin; factor VaRVVPLASMA, plasma-derived human factor V activated with RVV-V activator; factor VaIIa/CG, plasma-derived factor V activated with α-thrombin and treated with cathepsin G; factor VaIIa/NN, plasma-derived factor V activated with α-thrombin and treated with the purified enzyme from the venom of the snake Naja nigricollis nigricollis.
References
- Kalafatis M.; Egan J. O.; van’t Veer C.; Cawthern K. M.; Mann K. G. (1997) The regulation of clotting factors. Crit. Rev. Eukaryotic Gene Expression 7, 241–280. [DOI] [PubMed] [Google Scholar]
- Anderson P. J.; Nesset A.; Dharmawardana K. R.; Bock P. E. (2000) Role of proexosite I in factor Va-dependent substrate interactions of prothrombin activation. J. Biol. Chem. 275, 16435–16442. [DOI] [PubMed] [Google Scholar]
- Chen L.; Yang L.; Rezaie A. R. (2003) Proexosite-1 on prothrombin is a factor Va-dependent recognition site for the prothrombinase complex. J. Biol. Chem. 278, 27564–27569. [DOI] [PubMed] [Google Scholar]
- Heldebrant C. M.; Butkowski R. J.; Bajaj S. P.; Mann K. G. (1973) The activation of prothrombin. II. Partial reactions, physical and chemical characterization of the intermediates of activation. J. Biol. Chem. 248, 7149–7163. [PubMed] [Google Scholar]
- Esmon C. T.; Owen W. G.; Jackson C. M. (1974) A plausible mechanism for prothrombin activation by factor Xa, factor Va, phospholipid, and calcium ions. J. Biol. Chem. 249, 8045–8047. [PubMed] [Google Scholar]
- Rosing J.; Tans G.; Govers-Riemslag J. W.; Zwaal R. F.; Hemker H. C. (1980) The role of phospholipids and factor Va in the prothrombinase complex. J. Biol. Chem. 255, 274–283. [PubMed] [Google Scholar]
- Nesheim M. E.; Mann K. G. (1983) The kinetics and cofactor dependence of the two cleavages involved in prothrombin activation. J. Biol. Chem. 258, 5386–5391. [PubMed] [Google Scholar]
- Krishnaswamy S.; Church W. R.; Nesheim M. E.; Mann K. G. (1987) Activation of human prothrombin by human prothrombinase. Influence of factor Va on the reaction mechanism. J. Biol. Chem. 262, 3291–3299. [PubMed] [Google Scholar]
- Nesheim M. E.; Taswell J. B.; Mann K. G. (1979) The contribution of bovine factor V and factor Va to the activity of prothrombinase. J. Biol. Chem. 254, 10952–10962. [PubMed] [Google Scholar]
- Esmon C. T. (1979) The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J. Biol. Chem. 254, 964–973. [PubMed] [Google Scholar]
- Nesheim M. E.; Foster W. B.; Hewick R.; Mann K. G. (1984) Characterization of Factor V activation intermediates. J. Biol. Chem. 259, 3187–3196. [PubMed] [Google Scholar]
- Adams T. E.; Hockin M. F.; Mann K. G.; Everse S. J. (2004) The crystal structure of Activated Protein C-inactivated bovine factor Va: Implications for cofactor function. Proc. Natl. Acad. Sci. U.S.A. 101, 8918–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin G. L. (1990) Sulfation of tyrosine residues in coagulation factor V. Blood 76, 946–952. [PubMed] [Google Scholar]
- Armstrong S. A.; Husten E. J.; Esmon C. T.; Johnson A. E. (1990) The active site of membrane-bound meizothrombin. J. Biol. Chem. 265, 6210–6218. [PubMed] [Google Scholar]
- Esmon C. T.; Owen W. G.; Duiguid D. L.; Jackson C. M. (1973) The action of thrombin on blood clotting factor V: Conversion of factor V to a prothrombin-binding protein. Biochim. Biophys. Acta 310, 289–294. [DOI] [PubMed] [Google Scholar]
- Guinto E. R.; Esmon C. T. (1984) Loss of prothrombin and of factor Xa-factor Va interactions upon inactivation of factor Va by activated protein C. J. Biol. Chem. 259, 13986–13992. [PubMed] [Google Scholar]
- Luckow E. A.; Lyons D. A.; Ridgeway T. M.; Esmon C. T.; Laue T. M. (1989) Interaction of clotting factor V heavy chain with prothrombin and prethrombin 1 and role of activated protein C in regulating this interaction: Analysis by analytical ultracentrifugation. Biochemistry 28, 2348–2354. [DOI] [PubMed] [Google Scholar]
- Gerads I.; Tans G.; Yukelson L. Y.; Zwaal R. F.; Rosing J. (1992) Activation of bovine factor V by an activator purified from the venom of Naja naja oxiana. Toxicon 30, 1065–1079. [DOI] [PubMed] [Google Scholar]
- Bakker H. M.; Tans G.; Thomassen M. C. L. G. D.; Yukelson L. Y.; Ebberink R.; Hemker H. C.; Rosing J. (1994) Functional properties of human factor Va lacking Asp683-Arg709 domain of the heavy chain. J. Biol. Chem. 269, 20662–20667. [PubMed] [Google Scholar]
- Camire R. M.; Kalafatis M.; Tracy P. B. (1998) Proteolysis of factor V by cathepsin G and elastase indicates that cleavage at Arg1545 optimizes cofactor function by facilitating factor Xa binding. Biochemistry 37, 11896–11906. [DOI] [PubMed] [Google Scholar]
- Kalafatis M.; Beck D. O.; Mann K. G. (2003) Structural requirements for expression of factor Va activity. J. Biol. Chem. 278, 33550–33561. [DOI] [PubMed] [Google Scholar]
- Jenny R. J.; Pittman D. D.; Toole J. J.; Kriz R. W.; Aldape R. A.; Hewick R. M.; Kaufman R. J.; Mann K. G. (1987) Complete cDNA and derived amino acid sequence of human factor V. Proc. Natl. Acad. Sci. U.S.A. 84, 4846–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinto E. R.; Esmon C. T.; Mann K. G.; MacGillivray R. T. (1992) The complete cDNA sequence of bovine coagulation factor V. J. Biol. Chem. 267, 2971–2978. [PubMed] [Google Scholar]
- Heldebrant C. M.; Mann K. G. (1973) The activation of prothrombin. I. Isolation and preliminary characterization of intermediates. J. Biol. Chem. 248, 3642–3652. [PubMed] [Google Scholar]
- Franza B. R. J.; Aronson D. L.; Finlayson J. S. (1975) Activation of Human Prothrombin by a Procoagulant Fraction from the Venom of Echis carinatus. J. Biol. Chem. 250, 7057–7068. [PubMed] [Google Scholar]
- Morita T.; Iwanaga S.; Suzuki T. (1976) Activation of bovine prothrombin by an activator isolated from Echis carinatus venom. Thromb. Res. 8, Suppl. 259–65. [DOI] [PubMed] [Google Scholar]
- Kornalik F.; Blombäck B. (1975) Prothrombin activation induced by Ecarin: A prothrombin converting enzyme from Echis carinatus venom. Thromb. Res. 6, 57–63. [DOI] [PubMed] [Google Scholar]
- Myrmel K. H.; Lundblad R. L.; Mann K. G. (1976) Characteristics of the association between prothrombin fragment 2 and α-thrombin. Biochemistry 15, 1767–1773. [DOI] [PubMed] [Google Scholar]
- Doyle M. F.; Haley P. E. (1993) Meizothrombin: Active intemediate formed during prothrombin-catalyzed activation of prothrombin. Methods Enzymol. 222, 299–313. [DOI] [PubMed] [Google Scholar]
- Morita T.; Iwanaga S. (1981) Prothrombin activator from Echis carinatus venom. Methods Enzymol. 90, 303–311. [Google Scholar]
- Doyle M. F.; Mann K. G. (1990) Multiple active forms of thrombin. Relative activities of meizothrombins. J. Biol. Chem. 265, 10693–10701. [PubMed] [Google Scholar]
- Hibbard L. S.; Nesheim M. E.; Mann K. G. (1982) Progressive development of a thrombin inhibitor binding site. Biochemistry 21, 2285–2292. [DOI] [PubMed] [Google Scholar]
- Boxrud P. D.; Berliner L. J. (1996) Comparison of the active-site conformations of bovine α-thrombin and meizothrombin(desF1) by electron spin resonance. J. Protein Chem. 15, 231–242. [DOI] [PubMed] [Google Scholar]
- Martin P. D.; Malkowski M. G.; Box J.; Esmon C. T.; Edwards B. F. (1997) New insights into the regulation of the blood clotting cascade derived from the X-ray crystal structure of bovine meizothrombin des F1 in complex with PPACK. Structure 5, 1681–1693. [DOI] [PubMed] [Google Scholar]
- Beck D. O.; Bukys M. A.; Singh L. S.; Szabo K. A.; Kalafatis M. (2004) The contribution of amino acid region ASP695-TYR698 of factor V to procofactor activation and factor Va function. J. Biol. Chem. 279, 3084–3095. [DOI] [PubMed] [Google Scholar]
- Bukys M. A.; Kim P. Y.; Nesheim M. E.; Kalafatis M. (2006) A control switch for prothrombinase: Characterization of a hirudin-like pentapeptide from the COOH terminus of factor Va heavy chain that regulates the rate and pathway for prothrombin activation. J. Biol. Chem. 281, 39194–39204. [DOI] [PubMed] [Google Scholar]
- Toso R.; Camire R. M. (2006) Role of Hirudin-like factor Va heavy chain sequences in prothrombinase function. J. Biol. Chem. 281, 8773–8779. [DOI] [PubMed] [Google Scholar]
- Shim K.; Zhu H.; Westfield L. A.; Sadler J. E. (2004) A recombinant murine meizothrombin precursor, prtothrombin R157A/R268A, inhibits thrombosis in a model of acute carotide artery injury. Blood 104, 415–419. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E.; Katzmann J. A.; Tracy P. B.; Mann K. G. (1980) Factor V. Methods Enzymol. 80, 243–275. [DOI] [PubMed] [Google Scholar]
- Erdogan E.; Bukys M. A.; Kalafatis M. (2008) The contribution of amino acid residues 1508−1515 of factor V to light chain generation. J. Thromb. Haemostasis 6, 118–124. [DOI] [PubMed] [Google Scholar]
- Côté H. C. F.; Stevens W. K.; Bajzar L.; Banfield D. K.; Nesheim M. E.; MacGillivray T. A. (1994) Characterization of a stable form of human meizothrombin derived from recombinant prothrombin (R155A, R271A, and R284A). J. Biol. Chem. 269, 11374–11380. [PubMed] [Google Scholar]
- Barenholz Y.; Gibbs D.; Litmann B. J.; Goll J.; Thompson T.; Carlson D. (1977) A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry 16, 2806–2910. [DOI] [PubMed] [Google Scholar]
- Singh L. S.; Bukys M. A.; Beck D. O.; Kalafatis M. (2003) Amino acids Glu323, Tyr324, Glu330, and Val331 of factor Va heavy chain are essential for expression of cofactor activity. J. Biol. Chem. 278, 28335–28345. [DOI] [PubMed] [Google Scholar]
- Erdogan E.; Bukys M. A.; Orfeo T.; Mann K. G.; Kalafatis M. (2007) Identification of an inactivating cleavage site for α-thrombin on the heavy chain of factor Va. Thromb. Haemostasis 98, 998–1006. [PubMed] [Google Scholar]
- Bukys M. A.; Blum M. A.; Kim P. Y.; Brufatto N.; Nesheim M. E.; Kalafatis M. (2005) Incorporation of factor Va into prothrombinase is required for coordinated cleavage of prothrombin by factor Xa. J. Biol. Chem. 280, 27393–27401. [DOI] [PubMed] [Google Scholar]
- Kim P. Y.; Nesheim M. E. (2007) Further evidence for two functional forms of prothrombinase each specific for either of the two prothrombin activation cleavages. J. Biol. Chem. 282, 32568–32581. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970) Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Towbin H.; Staehlin T.; Gordon J. (1979) Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukys M. A.; Orban T.; Kim P. Y.; Beck D. O.; Nesheim M. E.; Kalafatis M. (2006) The structural integrity of anion binding exosite-I of thrombin is required and sufficient for timely cleavage and activation of factor V and factor VIII. J. Biol. Chem. 280, 27393–27401. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S.; Williams E. B.; Mann K. G. (1986) The binding of activated protein C to factors V and Va. J. Biol. Chem. 261, 9684–9693. [PubMed] [Google Scholar]