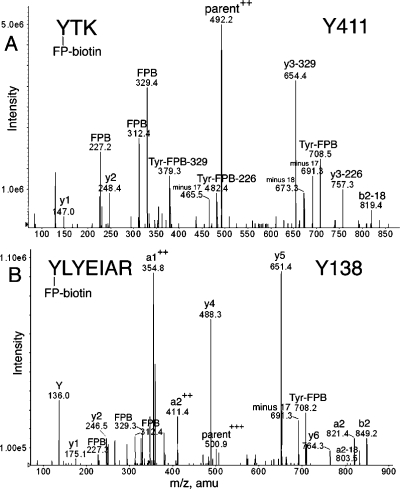
Figure 2.
MS/MS spectra of albumin peptides labeled with FP-biotin on Tyrosine. (A) Tyr 411 in peptide YTK and (B) Tyr 138 in peptide YLYEIAR are covalently modified by FP-biotin. The characteristic fragments of FP-biotin at 227.2, 312.4, and 329.3 amu are present. Ions characteristic of FP-biotin modification on tyrosine are the immonium ion at 708 amu and the immonium ion minus 17 at 691 amu. Support for modification of the first tyrosine rather than the second in YLYEIAR is the mass of ions a2, b2, and a2+2. The doubly charged parent ion in panel A had a mass of 492.2 amu. The triply charged parent ion in panel B had a mass of 500.9 m/z.