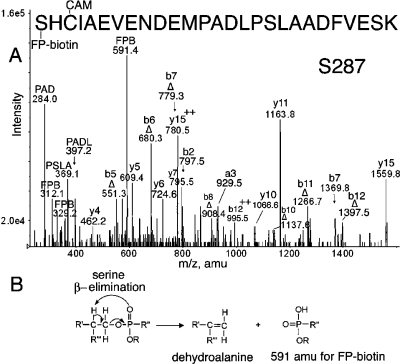
Figure 5.
(A) MS/MS spectrum of albumin peptide labeled with FP-biotin on Ser 287. The triply charged parent ion has a mass of 1183.8 m/z. The carbamidomethylated (CAM) peptide carried the FP-biotin on Ser 287. The evidence for modification on serine is the presence of a b-ion series for the dehydroalanine form of the peptide, designated Δ. The 591.4 amu ion is FP-biotin released from serine where the fluoride ion has been replaced by a hydroxyl group. Release of the entire OP accompanied by formation of dehydroalanine is a characteristic of OP bound to serine. Internal fragmentation at proline yielded masses at 284.0 for PAD, 369.1 for PSLA, and 397.2 for PADL. (B) Scheme for β-elimination of the OP label from serine. Fragmentation in the mass spectrometer eliminates the OP from serine and simultaneously converts serine to dehydroalanine.