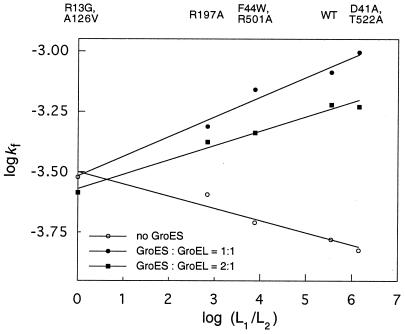
Figure 2.
Rates of GroEL-mediated folding of mDHFR as a function of the extent of negative cooperativity. Values of the rates of refolding (kf) of mDHFR, in the presence of different GroEL mutants and in the absence (○) or presence of 250 nM (●) or 500 nM (■) GroES oligomer, were determined as described in Experimental Procedures. Values of the allosteric equilibrium constants L1 (which equals [TR]/[TT]) and L2 (which equals [RR]/[TR]) were determined by fitting data of initial rates of ATPase activity as a function of ATP concentration as described (11). Values of logkf are plotted against the values of log(L1/L2), which are measures of the extent of negative cooperativity (11). The value of the correlation coefficient (r) is 0.99 for each of the three linear fits. The errors are up to 1% in kf and about 10% in log(L1/L2).