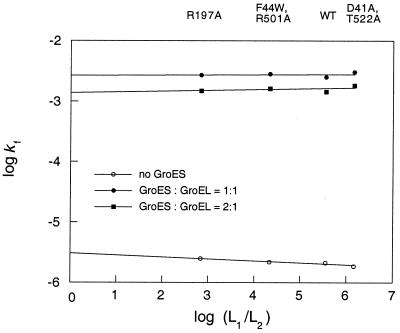
Figure 3.
Rates of GroEL-mediated folding of mitochondrial MDH as a function of the extent of negative cooperativity. Values of the rates of refolding (kf) of MDH, in the presence of different GroEL mutants and in the absence (○) or presence of 250 nM (●) or 500 nM (■) GroES oligomer, were determined as described in Experimental Procedures. Values of logkf are plotted against the values of log(L1/L2), which were calculated by fitting data of initial rates of ATPase activity as a function of ATP concentration as described (11). The errors are up to 10% in kf and about 10% in log(L1/L2).