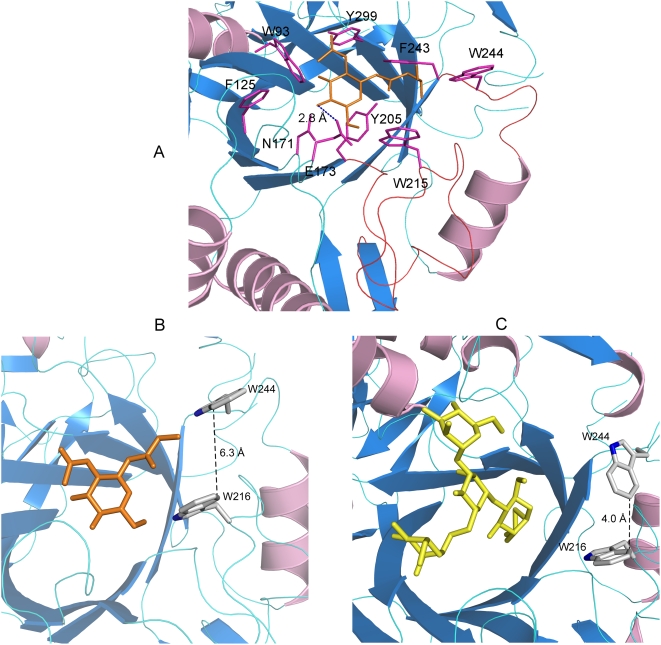
Figure 4. Active site of Endo-A.
(A) N171, E173 and Y299 are critical for catalysis. Amino acids surrounding the carbohydrate moiety are shown as sticks. The distances are shown in dashed lines. The GlcNAc-Asn moiety is shown as sticks. (B) W216 and W244 are “gate-keeping” the active site by sterically regulating access to the active site by the acceptor. The side chain of W244 moves during transglycosylation; the distance between the two Trp is much wider and allows passage of an acceptor into the active site. Trp and GlcNAc-Asn are shown as sticks. (C) The gate is “closed” in the structure of free protein (not shown) and Man3GlcNAc-thiazoline bound Endo-A (shown as sticks).