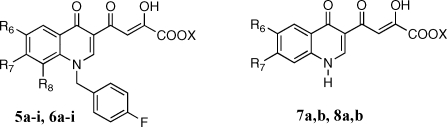
Table 2. Cytotoxicity, Antiviral, and Anti-Integrase Activities of Derivatives 5a−i, 6a−i, 7a,b, and 8a,b.
| anti-IN activity, IC50a |
antiviral activity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| compd | R6 | R7 | R8 | X | Mg2+ b | Mg2+ c | Mg2+ c | CC50d | EC50e | SIf |
| 5a | H | H | H | C2H5 | 0.67 | 0.79, 0.44 | 135 | 99 | 34.8 | 2.8 |
| 5b | F | H | H | C2H5 | 0.58 | 0.23, 0.44 | >333 | >200 | >50 | |
| 5c | H | F | H | C2H5 | 1.3 | 1.2, 2.9 | >333 | >200 | >50 | |
| 5d | H | H | F | C2H5 | 26 | 5.2 | >333 | >200 | >50 | |
| 5e | Cl | H | H | C2H5 | 21 | 3.0 | >333 | ntg | ntg | |
| 5f | H | Cl | H | C2H5 | 14 | 3.6 | >333 | ntg | ntg | |
| 5g | H | H | Cl | C2H5 | 32 | 5.2 | >333 | >200 | >50 | |
| 5h | Cl | Cl | H | C2H5 | 3.9 | 1.8 | 135 | 72 | >50 | |
| 5i | H | Pyh | H | C2H5 | 2.3 | 2.7 | 110 | >200 | 4.1 | 48.8 |
| 6a | H | H | H | H | 0.040 | 0.06, 0.16 | 14 | >200 | 1.17 | >171 |
| 6b | F | H | H | H | 0.030 | 0.14 | 2.3 | >200 | >50 | |
| 6c | H | F | H | H | 0.020 | 0.050 | 4.4 | >200 | >50 | |
| 6d | H | H | F | H | 0.018 | 0.16 | 16 | >200 | >50 | |
| 6e | Cl | H | H | H | 0.028 | 0.40 | 6.4 | >200 | 46.1 | 4.3 |
| 6f | H | Cl | H | H | 0.019 | 0.11 | 4.0 | >200 | >50 | |
| 6g | H | H | Cl | H | 0.023 | 0.51 | 44 | >200 | >50 | |
| 6h | Cl | Cl | H | H | 0.033 | 0.075 | 11, 34 | 156 | 3.2 | 62.5 |
| 6i | H | Pyh | H | H | 0.028 | 0.18 | 14, 9.0 | >200 | 0.17 | >1176 |
| 7a | Cl | H | C2H5 | 3.3 | 2.1 | 120 | ntg | ntg | ||
| 7b | H | Cl | C2H5 | >111 | 224 | >333 | ntg | ntg | ||
| 8a | Cl | H | H | >111 | 56 | 166 | ntg | ntg | ||
| 8b | H | Cl | H | 37 | 17 | >333 | ntg | ntg | ||
| 1i | 2.1 | |||||||||
| 2i | 0.05 | 1.0 | ||||||||
| 3i | 0.01 | 0.39j | ||||||||
| 4 | 0.016 | 0.017 | 0.44 | >200 | 4.29 | >47 | ||||
Inhibitory concentration 50% (μM) determined from dose response curves.
Experiments performed in duplicate using the high throughput electrochemiluminescent (HTECL) assay (ST assay in the presence of Mg2+).
Experiments performed in a gel-based assay in the presence of Mg2+ (see Figure 3).
Cytotoxic concentration 50% (μM).
Effective concentration 50% (μM).
Selectivity index = CC50/EC50.
nt: not tested.
Py = 1-pyrrolidinyl.
This data is referred to EC95.