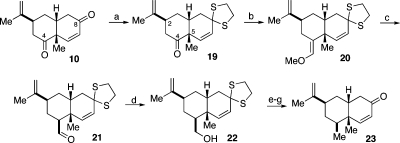
Scheme 3.
Reagents and conditions: (a) 1,2-ethanedithiol, MeOH, BF3·OEt2, 0 °C, 30 h, 83%; (b) Ph3PCH2OCH3Cl, KN(SiMe3)2, THF, −30 to 0 °C to rt, 24 h; (c) 4 N HCl, MeOH, THF, 0 °C to rt, 36 h, 89% over two steps, 13:1 β:α; (d) NaBH4, MeOH, THF, 0 °C to rt, 2 h, 90%; (e) MsCl, Et3N, CH2Cl2, 0 °C to rt, 1.5 h; (f) LiBHEt3, THF, 0 °C to rt, 24 h, 71% over two steps; (g) (CF3CO2)2IPh, MeOH, H2O, CH2Cl2, rt, 15 min, 87%.