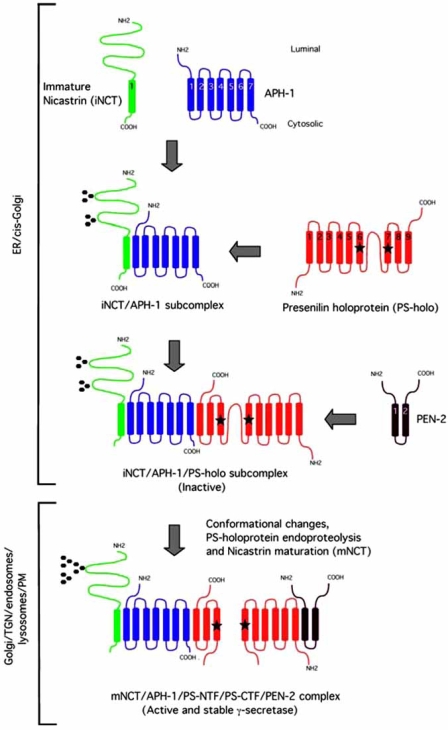
Fig. (3).
Schematic representation of the assembly and activation in the secretory pathway of the γ-secretase complex. The assembly of the γ-secretase complex involves the early formation in the ER of an intermediate subcomplex of APH-1 and the “immature” N-linked isoform of NCT (iNCT) that is not associated with active γ-secretase (= iNCT/APH-1 subcomplex). PS-holoprotein then binds to the iNCT/APH-1 subcomplex to form a second trimeric intermediate (= iNCT/APH-1/PS-holoprotein subcomplex). In this trimeric intermediate, PS-holoprotein likely adopts a conformation in which the hydrophobic domain that contains the site of PS-holoprotein endoproteolysis is membrane-embedded in a close proximity to TMD6 and TMD7, with the consequence of occluding the active site. The association of PEN-2 in the ER/cis-Golgi compartments to the trimeric and inactive iNCT/APH-1/PS-holoprotein subcomplex triggers a conformational change (in PS-holoprotein?) that promotes (1) the endoproteolytic cleavage of PS-holoprotein and subsequently (2) the activation and trafficking of γ-secretase, with (3) the conversion from an immature to a mature form of NCT (mNCT) with a different and more complex glycosylation pattern. The fully matured and active γ-secretase (mNCT/APH-1/PS-NTF/PS-CTF/PEN-2) is mainly localized in different cell compartments including the Golgi, the trans-Golgi network (TGN), the endosomes, the lysosomes and the plasma membrane.