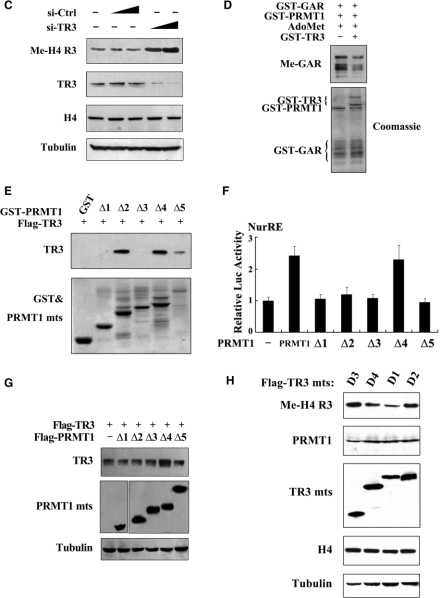
Figure 4.
TR3 inhibits PRMT1 methyltransferase activity. (A) Full-length PRMT1 but not its truncation mutants methylate H4 R3 in vitro. GST-PRMT1 and its mutants (Top) were incubated with Histone and AdoMet, respectively. The reaction mixtures were resolved on SDS-PAGE, and visualized by anti-methyl-H4 R3 antibody. (B, C) TR3 exerted an inhibitory effect on the methylation of H4 R3 in vivo. Myc-TR3 (B) or siRNA-TR3 (C) was transfected into 293T cells with or without PRMT1. The in vivo methylation of H4 R3 was examined. (D) Effect of TR3 on the in vitro methylation of GAR by PRMT1. The methylation of GAR in vitro was determined with anti-dimethyl-arginine asymmetric antibody (ASYM24). (E) TR3 interacts with PRMT1 truncation mutants. Different GST-PRMT1 mutants were separately incubated with Flag-TR3 protein immunoprecipitated from 293T cells to detect their interactions by GST pull-down assay. (F) Effect of PRMT1 mutants on TR3 transactivational activity. Different PRMT1 truncation mutants were transfected into 293T cells. The luciferase activity of NurRE reporter gene was determined. (G) Effect of PRMT1 mutants on TR3 expression levels. Different PRMT1 truncation mutants together with Flag-TR3 were transfected into 293T cells. The expression levels of TR3 were determined by western blotting. (H) Effect of TR3 mutants on PRMT1-mediated H4 R3 methylation. Different TR3 mutants as indicated were transfected into 293T cells. The in vivo methylation of H4 R3 was examined.