Figure 3.
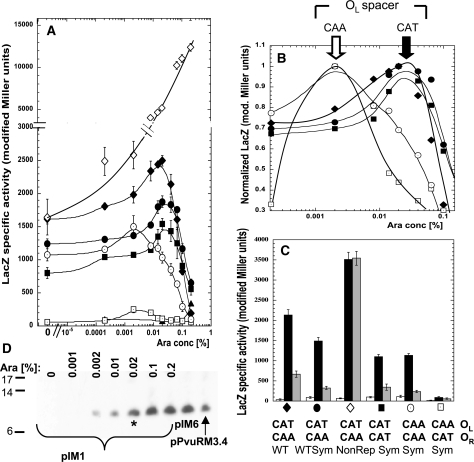
Effect of exchanging or duplicating the native intra-operator spacers. (A) C.PvuII titration experiment performed as in Figure 2C. Closed symbols represent LacZ activity from PpvuIICR–lacZ fusions with, respectively, the native PvuII C-boxes with CAT/CAA intra-operator spacers (filled diamonds, pDK435), the symmetrized C-box variant with native spacers (filled circles, pIM8), the symmetrized variant with CAA/CAA spacers (open circles, pIM13), with CAT/CAT spacers (filled squares, pIM10) and with CAA/CAT spacers (open squares, pIM14). The nonrepressing C-box mutant (pWWWR) was added as control (open diamonds). All cells contain a compatible plasmid carrying PBAD–pvuIIC (pIM1). As C.PvuII negative control pBAD24 was used with pDK435 (triangles). (B) C.PvuII concentrations giving peak expression. Same data as shown in (A), but normalized to the highest expression value (LacZ modified Miller units) for each variant. (C) Same plasmids used in (A), but with C.PvuII delivered at a physiological level from the WT C-box/promoter in the intact R-M system (pPvuRM3.4, black bars), or elevated level from a nonrepressing C-box mutant of R-M system (pIM6, gray bars). A plasmid with no C.PvuII gene was used as a negative control (pBR322, white bars). The symbols at the bottom facilitate comparison to (A) and (B). (D) Arabinose-induced WT C.PvuII production visualized by western blotting. Plasmid pIM1 (also used in A) was in E. coli cells growing in the indicated arabinose concentration. The level of C.PvuII produced from pPvuRM3.4 and its nonrepressing variant (pIM6) were also determined. The asterisk indicates the C.PvuII level at 0.02% arabinose induction, corresponding to the highest level of PpvuIICR–lacZ transcription [peak for black diamonds in (A)].