Table 1.
Reaction of imine 1 with p-cresol.a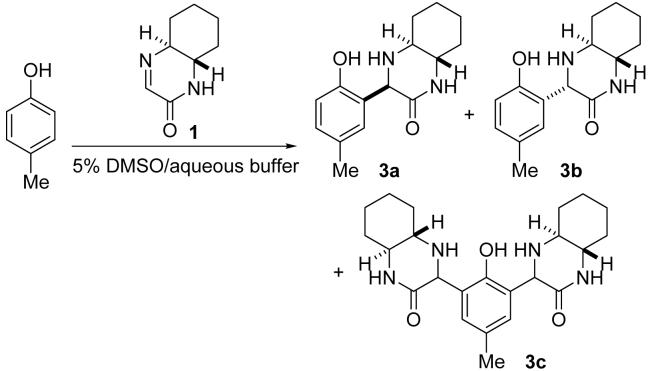
| entry | aqueous buffer (aqueous component) | yieldb (%) | 3a:3bc |
|---|---|---|---|
| 1 | 200 mM NaH2PO4, pH 5 | 55 | 1.3:1 |
| 2 | 200 mM NaH2PO4-Na2HPO4, pH 6.0 | 36 (20) | 1.2:1 |
| 3 | 200 mM NaH2PO4-Na2HPO4, pH 7.0 | 44 | 2.0:1 |
| 4 | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 46 | 3.3:1 |
| 5d | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 50 | 2.5:1 |
| 6e | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 52 | 2.5:1 |
| 7f | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 53 | 2.8:1 |
| 8 | 200 mM Na2HPO4, pH 9 | 44 | 3.9:1 |
| 9 | PBS, pH 7.4g | 41 | 2.3:1 |
| 10 | 100 mM Tris, pH 8.0g | 41 | 3.2:1 |
| 11 | 25 mM HEPES, pH7.5g | 50 | 2.8:1 |
| 12 | 10% CH3COOH, pH 2 | 24 | 0.8:1 |
| 13 | H2O | 36 | 2.7:1 |
| 14 | 200 mM Na2CO3, pH 10 | 49 | 2.1:1 |
| 15 | 200 mM Et3N, pH 10 | 29 | 2.1:1 |
| 16h | 100 mM NaH2PO4, pH 5 | 23 (60) [143] | 1.3:1 |
| 17h | 100 mM NaH2PO4-Na2HPO4, pH 6 | 31 (48) [127] | 1.5:1 |
| 18h | 100 mM NaH2PO4-Na2HPO4, pH 7 | 39 (27) [93] | 2.0:1 |
| 19h | 100 mM Na2HPO4,pH 9 | [76] | ndi |
| 20j | 100 mM NaH2PO4-Na2HPO4, pH 7 | 32 (45) | 2.0:1 |
Conditions: Imine 1 (95 mM) and p-cresol (25 mM) in 5% DMSO/indicated aqueous buffer (or aqueous component) at 23 °C for 24 h.
Sum of 3a and 3b; yield of 3c is indicated in parenthesis; determined by 1H NMR of the extracted mixture. For entries 1-15, yield of 3c was <10% except where noted. Percentage of modified ortho positions of the phenolic OH of p-cresol is indicated in bracket; complete conversion to 3c = 200% in bracket.
Determined by 1H NMR of the extracted mixture.
Imine 1 (95 mM) and p-cresol (5 mM).
Imine 1 (25 mM) and p-cresol (500 μM).
Imine 1 (5 mM) and p-cresol (500 μM).
PBS = phosphate buffered saline. Tris = tris(hydroxymethyl)aminomethane. HEPES= 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.
Imine 1 (50 mM) and p-cresol (10 mM) in indicated buffer prepared with D2O (no DMSO was added) at 37 °C for 24 h. Yield was determined by 1H NMR of the reaction mixture.
Not determined.
Imine 1 (50 mM) and p-cresol (10 mM) in the absence of DMSO at 37 °C.
