Table 2.
Reaction of imine 2 with p-cresol.a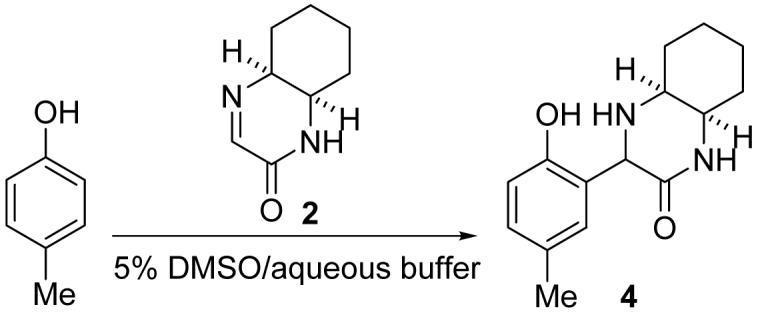
| entry | aqueous buffer | yieldb (%) |
|---|---|---|
| 1 | 200 mM NaH2PO4, pH 5 | 37 |
| 2 | 200 mM NaH2PO4-Na2HPO4, pH 6.0 | 35 |
| 3 | 200 mM NaH2PO4-Na2HPO4, pH 7.0 | 32 |
| 4 | 200 mM NaH2PO4-Na2HPO4, pH 8.0 | 26 |
| 5 | 200 mM Na2HPO4, pH 9 | 22 |
| 6 | 100 mM Tris, pH 8.0 | 25 |
| 7c | 100 mM NaH2PO4-Na2HPO4, pH 7 | [68] |
Imine 2 (95 mM) and p-cresol (25 mM) at 23 °C for 24 h.
Determined by 1H NMR of the extracted mixture. For entries 1-6, yield of di-addition product was <5%. Percentage of modified ortho positions of the phenolic OH of p-cresol is indicated in bracket; complete conversion to the di-addition product = 200% in bracket.
Imine 2 (50 mM) and p-cresol (10 mM) in the buffer prepared with D2O (no DMSO was added) at 37 °C for 24 h.
