Abstract
Background
Cannabinoids induce analgesia by acting on cannabinoid receptor (CBR) types 1 and/or 2. However, central nervous system side effects and antinociceptive tolerance from CBR1 limit their clinical use. CBR2 exist on spinal glia and perivascular cells, suggesting an immunoregulatory role of these receptors in the central nervous system. Previously, the authors showed that spinal CBR2 activation reduces paw incision hypersensitivity and glial activation. This study tested whether CBR2 are expressed in glia and whether their activation would induce antinociception, glial inhibition, central side effects, and antinociceptive tolerance in a neuropathic rodent pain model.
Methods
Rats underwent L5 spinal nerve transection or sham surgery, and CBR2 expression and cell localization were assessed by immunohistochemistry. Animals received intrathecal injections of CBR agonists and antagonists, and mechanical withdrawal thresholds and behavioral side effects were assessed.
Results
Peripheral nerve transection induced hypersensitivity, increased expression of CR3/CD11b and CBR2, and reduced ED2/CD163 expression in the spinal cord. The CBR2 were localized to microglia and perivascular cells. Intrathecal JWH015 reduced peripheral nerve injury hypersensitivity and CR3/CD11b expression and increased ED2/CD163 expression in a dose-dependent fashion. These effects were prevented by intrathecal administration of the CBR2 antagonist (AM630) but not the CBR1 antagonist (AM281). JWH015 did not cause behavioral side effects. Chronic intrathecal JWH015 treatment did not induce antinociceptive tolerance.
Conclusions
These data indicate that intrathecal CBR2 agonists may provide analgesia by modulating the spinal immune response and microglial function in chronic pain conditions without inducing tolerance and neurologic side effects.
NEUROPATHIC pain, resulting from nerve injury, is often the most difficult pain to treat. Gabapentin, tramadol, local lidocaine patches, opioids, and tricyclic anti-depressants are first-line medications for neuropathic pain. However, their modest effectiveness, physical dependence, significant side effects, or lack of universal efficacy limit their clinical use.1 Therefore, new strategies for better treatment of pain are needed. Cannabinoids are potential analgesic agents and are thought to exert most of their effects by binding to G protein-coupled cannabinoid receptor (CBR) types 1 and 2. CBR1 exist in neural structures and are expressed in brain, spinal cord, and peripheral nerves.2-6 Alternatively, CBR2 are expressed in immune cells7 and keratinocytes8 and have recently been shown to exist in the central nervous system (CNS).9,10 In accord with the role of CBR2 in immune cells, CBR2 are expressed in microglia and perivascular cells in normal human and rat brain11,12 and in microglia and astrocytes, especially during inflammation.13,14 These findings suggest that the cannabinoid system may have immune modulatory functions also in the CNS. Microglial and astrocytic activation, defined as an increase in the expression of specific surface antigens and functional cellular proteins, is involved in the initiation and maintenance of hypersensitivity in neuropathic pain.15-18 Peripheral nerve injury induces CBR2 expression in peripheral fibers and spinal cord.19-21 In addition, although CBR2 are presumably increased in microglia after peripheral nerve injury,20 no conclusive CBR2 expression in glial cells has been shown in vivo thus far.
Central CBR1 activation induces antinociception in several pain models, but neurologic side effects and antinociceptive tolerance limit its clinical use as a potential analgesic. In spite of this, CBR1 have been extensively studied, whereas the function of spinal cord CBR2 has not been fully described in the processing of nociceptive information.22 We have previously demonstrated that spinal CBR2 are functional and that their activation reduces paw incision-induced pain in association with a reduction of microglial and astrocytic activation without inducing neurologic side effects. Herein, we test the hypothesis that CBR2 are expressed in glial cells after peripheral nerve injury and that their activation reduces peripheral nerve injury-induced microglial activation and hypersensitivity. Because chronic activation of spinal CBR2 in neuropathic pain has not been assessed, a second hypothesis was tested in the current study, that chronic spinal CBR2 activation does not induce antinociceptive tolerance.
Materials and Methods
Animals and Surgeries
After approval by the Institutional Animal Care and Use Committee at Dartmouth College (Dartmouth Medical School, Hanover, New Hampshire) and in accordance with the Guidelines for Animal Experimentation of the International Association for the Study of Pain, male Sprague-Dawley rats weighing 250-300 g (Harlan, Indianapolis, IN) at the start of surgery underwent L5 nerve transection (L5NT) surgery as previous described.23 Briefly, rats were anesthetized with 2% isoflurane in oxygen, and a small incision to the skin overlying L5-S1 was made, followed by retraction of the paravertebral musculature from the vertebral transverse processes. The L6 transverse process was then partially removed to expose the L4 and L5 spinal nerves. The L5 spinal nerve was identified, lifted slightly, and transected. The wound was irrigated with saline and sutured in two layers. The sham surgery consisted of exposure of the L5 spinal nerve without transection. Animals were housed individually and maintained in a 12:12 h light-dark cycle with ad libitum access to food and water. Efforts were made to limit animal distress and to use the minimum number of animals necessary to achieve statistical significance.
Behavioral Testing
The 50% withdrawal threshold to mechanical stimuli was measured twice at 5- to 10-min intervals ipsilaterally to surgery using calibrated von Frey filaments (Stoelting, Wood Dale, IL) and an up-down statistical method.24 The average of these values was used for data analyses. The withdrawal threshold was determined for each animal before surgery, 1 and 4 days after surgery (immediately before any pharmacologic treatment), and after drug administration (different time points for different paradigms; see Acute Antinociceptive Effect Study, Spinal CBR2 Activation-induced Hypersensitivity Study, and Spinal CBR2 Activation-induced Tolerance Study sections). The investigator was blinded to drug treatment in all behavioral tests. Withdrawal thresholds were converted to the percentage of maximum possible effect according to the formula (withdrawal threshold after drug - withdrawal threshold 24 h after surgery) × 100/(withdrawal threshold before surgery - withdrawal threshold 24 h after surgery).
Drugs and Treatments
The drugs used were as follows: the nonselective cannabinoid receptor agonist CP55940 (5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol; Sigma Chemical Co., St. Louis, MO), the CBR1 antagonist AM281 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide), the CBR2 antagonist AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone), and the CBR2 agonist JWH015 ((2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenylmethanone), purchased from Tocris (Ellisville, MI). Drugs were diluted in dimethylsulfoxide and saline in a ratio of 1:1 and administered in a volume of 15 μl. Drugs were administered by intrathecal injections by means of lumbar puncture under brief inhalational anesthesia (2-4% isoflurane in oxygen) using a Hamilton syringe and a 28-gauge ⅝-in hypodermic needle. The needle was inserted intrathecally, on the midline between the fourth and fifth lumbar vertebrae. The correct injection site was confirmed with the stimulation of nerves in the cauda equina when the needle penetrated the dura and manifested with a brief but obvious movement of the tail and/or the hind paws. The animals regained consciousness 2-3 min after the discontinuation of anesthesia.
Acute Antinociceptive Effect Study
Four days after surgery, drugs were administered in two injections at a 2-h interval. A dose response of JWH015 was performed in four different groups: 0.4, 1, 2, and 10 μg/injection (n = 5, 5, 7, and 10, respectively). A group was treated with the nonselective CBR agonist CP55940 (10 μg/injection, n = 6). To challenge the antinociceptive effect of JWH015 (10 μg/injection), one group received AM281 (CBR1 antagonist, 10 μg/injection, n = 7) and the other received AM630 (CBR2 antagonist, 10 μg/injection, n = 7) concomitantly with JWH015. Sham (n = 11) and L5NT (n = 7) groups received vehicle injections and were used as the controls. Another sham surgery group was treated with JWH015 (10 μg/injection, n = 5) to test possible effects in nonsensitized animals. Behavioral testing was performed 15 min, 30 min, 1 h, and 2 h after each injection.
Spinal CBR2 Activation-induced Hypersensitivity Study
The same paradigm used in the acute antinociceptive effect study was used for 5 consecutive days in four different groups. Either JWH015 (10 μg/injection) or vehicle was chronically administered (two 2-h interval injections daily, starting at 8:00 -9:00 am) in L5NT (n = 8 for JWH015 and n = 7 for vehicle) or sham animals (n = 5 for JWH015 and n = 3 for vehicle). Behavioral testing was performed 15 min and 2 h after the first injection and 15 min, 2 h, and 24 h after the second injection.
Spinal CBR2 Activation-induced Tolerance Study
Intrathecal JWH015 was acutely administered in 30-min interval escalating doses: 0.4, 2, 10, and 50 μg in L5NT and sham animals that had previously received chronic (5 days) treatment with JWH015 (n = 8 for L5NT and n = 4 for sham) or vehicle (n = 5 for L5NT and n = 3 for sham). Experiments were performed 24 h after the last day of chronic treatment. The antinociceptive effect of escalating doses of JWH015 was studied 15 min after every injection, and its effectiveness and potency were compared in both chronic JWH015 and chronic vehicle treatment groups.
Motor Function and Reflex Testing
Based on previous studies, righting and placing-stepping tests were used to evaluate motor reflexes,25 and the bar test was used to evaluate catalepsy.26 Because CBR agonists have also been noted to induce vocalization (as a sign of irritability, hypersensitivity, or pain) and reduce exploratory activity,27 these were also evaluated. The placing-stepping reflex was tested by placing the rostral aspect of the hind paws on the edge of a table and was quantified as the seconds in which the animals put the paws up and forward into a position to walk. A cutoff of 60 s was used. The bar test consists of placing the forelimbs on a bar of approximately 1 cm in diameter and 10 cm above and parallel to a table, leaving the hind paws resting on the table. A cataleptic animal will stay in that position longer than a normal animal. The time in which the animal puts its forelimb on the table was recorded, using a cutoff time of 60 s. The righting test consists of placing the animal prone and recording the ability to right itself, studied as normal (an immediate and coordinated twisting of the body to an upright position), mild (ability to completely right, but slowly), moderate (ability to right the forelimbs slowly followed by the hind limbs with more difficulty), and severe impairment (inability to right in 20 s). Vocalization was rated as absent, present sometimes when manipulated, always present when manipulated, or present with even light touch. Exploratory activity was rated as normal, only head movements without vertical and/or horizontal exploration, or no spontaneous movements or splayed posture with no spontaneous movements. A scale of 0 -3, from normal to severe impairment, was chosen to evaluate these parameters. All behavioral measures were performed twice, and the average was used for analyses.
Tissue Preparation and Immunohistochemistry
Rats were deeply anesthetized with 2-4% isoflurane in oxygen and perfused transcardially with buffer (0.01 m phosphate-buffered saline, 150 ml) followed by 4% formaldehyde (350 ml) at room temperature. The L5 portion of the spinal cord was removed and placed in 30% sucrose for 48-72 h at 4°C. The tissue was then frozen at -80°C in optimal cutting temperature compound (Sakura Finetek, Torrance, CA). Immunohistochemistry was performed on transverse 20-μm L5 spinal cord free-floating sections by using the Vector ELITE ABC (Vector Labs, Burlingame, CA), avidin- biotin complex technique. A mouse monoclonal antibody for CR3/CD11b (1:2; gift from William F. Hickey, M.D., Professor of Pathology, Department of Pathology, Dartmouth Medical School, Hanover, NH) was used to label the expression of CR3/CD11b on microglia, and a rabbit polyclonal antibody for glial fibrillary acidic protein (GFAP) was used to label astrocytes (1:10,000; Dako Cytomation, Glostrup, Denmark). A goat polyclonal antibody against the C terminus of CBR2 was used to label CBR2 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA; sc10076). This CBR2 antibody and concentration were chosen based on its specificity. Specificity was tested by omitting the primary antibody and by the degree of nonspecific background staining. Other commercial antibodies were tested in parallel at different concentrations and were found to display nonspecific staining. The sections were examined with an Olympus microscope, and images were captured with a Q-Fire cooled camera (Olympus, Melville, NY). A monoclonal antibody and immunofluorescence was used to label the expression of ED2/CD163 (1:150; Serotec, Raleigh, NC) on perivascular cells.
Glial activation has previously been determined by comparing immunofluorescence staining intensity.28,29 Herein, we quantified the ED2/CD163, CR3/CD11b, and GFAP staining, blinded to experimental conditions, as the number of pixels above a preset intensity threshold using SigmaScan Pro 5 (SPSS, Chicago, IL) as previously described.28 Glial activation is characterized for an increase in the number (proliferation), migration, and complexity of these cells (rounded cell bodies and thicker processes), resulting in an increase in labeling. Therefore, an increase in the number of pixels was interpreted as a sign of glial activation. Rats on postoperative day 4 and 2 h after intrathecal vehicle (n = 4 for sham and n = 3 for L5NT), 2 μg JWH015 (n = 3), or 10 μg JWH015 (n = 4) were used to determine microglial or astrocytic activation or ED2/CD163 expression (n = 4 for all groups) induced by L5NT and to study the effects of JWH015 on glial activation. A different group of sections were used to evaluate the effects of the antagonists (AM630 n = 5, AM281 n = 7 for microglia, and AM630 n = 4, AM281 n = 3 for ED2/CD163) on JWH015 effects (n = 8 for microglia and n = 4 for ED2/CD163). Data were normalized as percentage of sham group to compare L5NT and JWH015 effects or as percentage of JWH015 (10 μg) to compare the effects of the antagonists.
To quantify the expression of CBR2 (n = 3 for sham and L5NT groups), the same methodology was used. The data were normalized as percentage of contralateral side to sham surgery. For both microglial and astrocytic activation and CBR2 expression, the staining intensity was examined in a standardized area of laminae I-II with three or four slices examined per animal. ED2/CD163 expression was quantified as the number of positive staining cells in L5 dorsal horn spinal cord with three or four slices examined per animal. To study the localization of CBR2, immunofluorescence was performed using the same CBR2 antibody plus: Iba1 (microglia marker, rabbit polyclonal, 1:500; Wako Pure Chemical Industries, Richmond, VA), GFAP (astrocyte marker), S100B (astrocyte marker, rabbit polyclonal, 1:15,000; Fitzgerald, Concord, MA), ED2 (CD163 glycoprotein, perivascular cell marker, 1:150, mouse immunoglobulin [Ig] G1 anti-rat CD163; Serotec), or NeuN (neuron marker, 1:10,000 mouse IgG1 anti-NeuN; Chemicon, Temecula, CA). After three washes in phosphate buffer solution, spinal cord sections were incubated in 3% fetal bovine serum-phosphate buffer solution for 30 min at room temperature. Primary antibodies were applied in the following combinations overnight at 4°C: goat anti-CBR2 and mouse anti-ED2; goat anti-CBR2 and rabbit Iba1; goat anti-CBR2 and mouse anti-NeuN; goat anti-CBR2 and rabbit anti-GFAP; goat anti-CBR2 and rabbit anti-S100B; or goat anti-CBR2, rabbit anti-GFAP, and mouse anti-ED2. The following day, tissue sections were washed and then visualized with the appropriate secondary fluorescent antibody (Molecular Probes, Invitrogen, Carlsbad, CA): chicken anti-goat Alexa Fluor-647 (1:1,000), goat anti-mouse IgG1 Alexa Fluor-488 (1:250), goat anti-rabbit IgG Alexa Fluor-488, goat antirabbit IgG Alexa Fluor-405 (1:250). Secondary antibody staining was performed in two steps to avoid cross-reaction: (1) incubation with chicken anti-goat Alexa Fluor-647 (1 h at room temperature) followed by two washes and (2) incubation with goat anti-rabbit IgG Alexa Fluor-488 and/or goat anti-mouse IgG1 Alexa Fluor-488 (1 h at room temperature).
Finally, tissue sections were washed and mounted with Vectashield (Vector Labs) containing 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; Sigma) to visualize cell nuclei. The sections were examined with an Olympus fluorescence microscope, and images were captured with a Q-Fire cooled camera (Olympus). Confocal microscopy was also performed using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss AG, Oberkochen, Germany; Englert Cell Analysis Laboratory, Dartmouth). Merged color images were processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). The tissues used to study microglial and astrocytic activation, ED2/CD163 expression, and CBR2 and expression and localization were harvested from the same animals that were tested for behavioral data.
Statistical Analyses
The effects of L5NT, sham surgery, and drug injections on bar test and placing-stepping test were determined using a repeated-measures two-way analysis of variance followed by the Bonferroni posttest using after-surgery data as control. The effects of L5NT, sham surgery, and drug injections on withdrawal thresholds were examined using the Friedman repeated-measures analysis of variance on rank test. If significant effects were found, nonparametric Wilcoxon signed ranks tests were conducted comparing each time point to the threshold on day 4 after surgery. Between-group differences were examined at each time period using the Kruskal-Wallis test. Significant effects were followed using the Mann-Whitney U test comparing only the novel treatment to control or agonist group. At 2 h after treatment time point, the dose of JWH015 producing 50% of maximum efficacy (ED50) and its 95% confidence limits were calculated. In the tolerance study, acute intrathecal JWH015 ED50 was compared between chronic JWH015 and chronic vehicle groups using a t test. Comparisons among groups for ED2/CD163 (number of cells), CBR2, and microglial or astrocytic staining (in pixels) were performed using t tests or, when normality failed, Mann-Whitney U test. Data are presented as mean ± SEM. Vocalization and exploratory activity data after the treatment were pooled in each group and compared; differences were examined using the Kruskal-Wallis test. Data are presented as median ±95% confidence limits. In all cases, a P value less than 0.05 was considered significant. SigmaStat (Systat Software, San Jose, CA) and GraphPad inStat (GraphPad Software, San Diego, CA) software were used.
Results
L5NT-induced Spinal CBR2 Expression
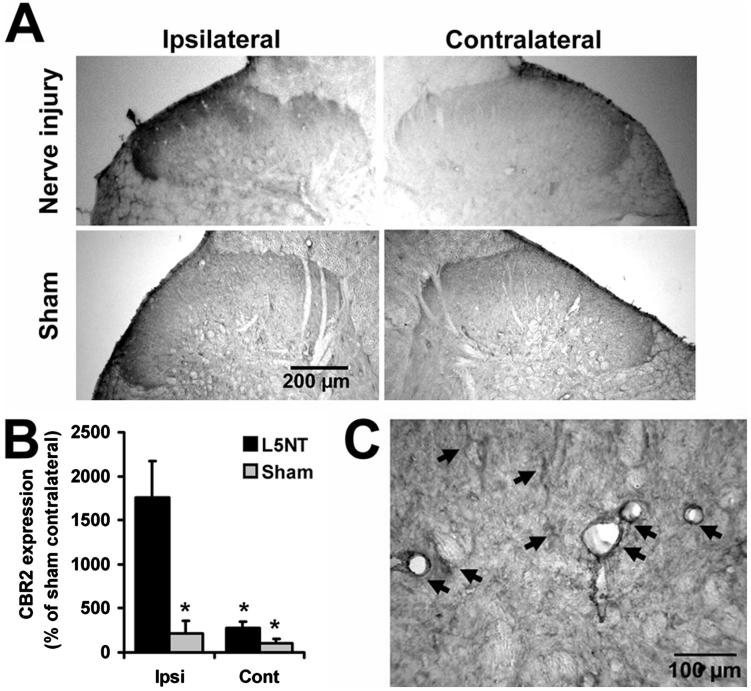
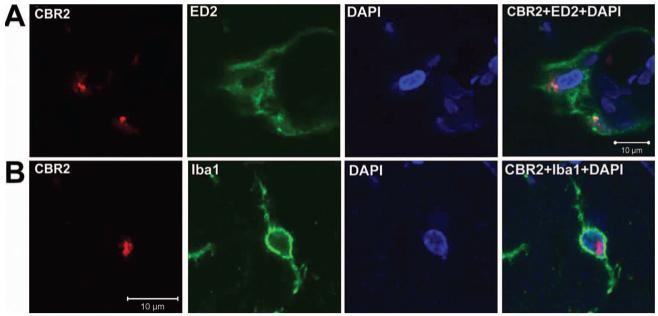
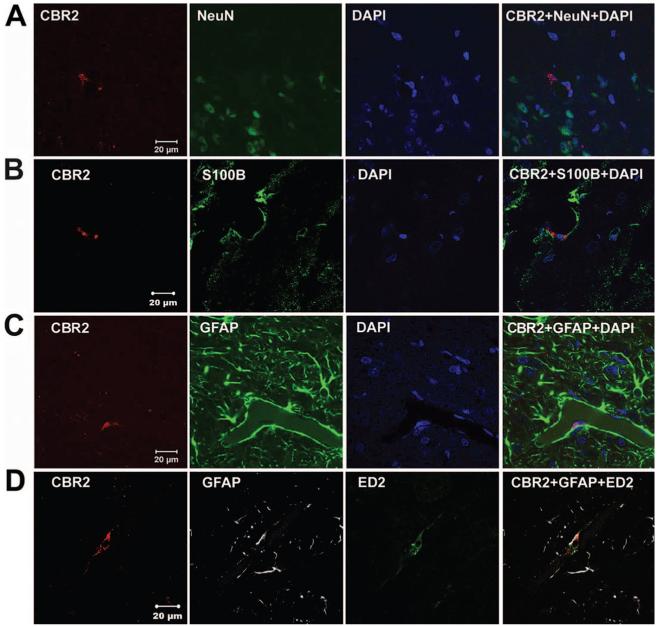
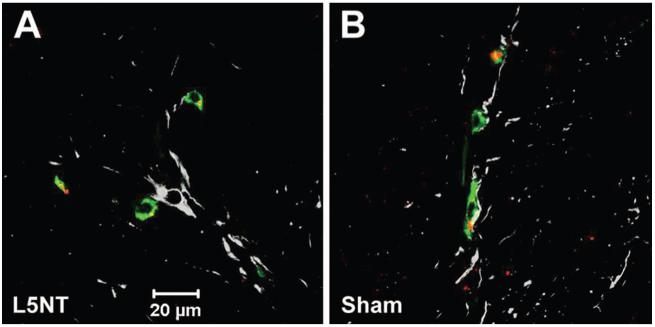
CBR2-like staining intensity was enhanced in the dorsal horn ipsilateral to the injury 4 days after L5NT compared with the contralateral side to L5NT and ipsilateral and contralateral side to sham surgery (P < 0.05). This enhanced expression of CBR2 was obvious in superficial laminae (fig. 1A). Intensity of CBR2-like staining in the dorsal horn ipsilateral to sham surgery was not significantly different from the dorsal horn contralateral to sham surgery (fig. 1B). CBR2-like staining was found in cells with microglial morphology and perivascular cells (fig. 1C). To confirm these findings, immunofluorescence colabeling and confocal imaging were performed. CBR2-positive cells were coregionalized with either ED2/CD163 (perivascular cells, fig. 2A)- or Iba1 (microglia, fig. 2B)-positive cells in the dorsal horn ipsilateral but also contralateral to L5NT or sham surgery. This coregionalization was observed in the nuclei of some cells (fig. 2B) but also in the cell membrane as punctuate staining (data not shown). No coregionalization of CBR2 with NeuN (neurons, fig. 3A) was observed. CBR2 staining was observed adjacent to both astrocyte markers used, S100B and GFAP (figs. 3B and C), but apparent coregionalization was not observed. To further confirm this, we performed triple staining using ED2/CD163, GFAP, and CBR2 antibody markers (figs. 3D and 4). This staining demonstrated that CBR2 expression was close but not coregionalized to GFAP containing cells (astrocytes). However, GFAP-positive cells were found in intimate contact with perivascular-like cells (ED2/CD163) expressing CBR2 (figs. 3D and 4). Most of ED2/CD163-positive cells were found to express CBR2 in spinal cord dorsal horn ipsilateral to L5NT; however, this was observed to a lesser extent in spinal cord dorsal horn ipsilateral to sham surgery (fig. 4).
Fig. 1.
Representative images of cannabinoid receptor type 2 (CBR2) staining. (A) Dorsal horns of L5 nerve transection (nerve injury, upper panel) or sham-operated animals (lower panel) after intrathecal vehicle. (B) CBR2 staining quantification of laminae I and II of nerve injury (n = 3) or sham (n = 3) animals represented as percentage of the staining intensity of superficial dorsal horn contralateral (Cont) to sham surgery. * P < 0.05 compared with ipsilateral (Ipsi) to nerve injury surgery (t test). L5NT = L5 nerve transection. (C) Representative image of CBR2 staining showing microglia-like and perivascular-like morphologies (arrows).
Fig. 2.
Cannabinoid receptor type 2 (CBR2) staining and localization. Representative confocal images of CBR2 (red), ED2/CD163 (ED2; green) and 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; blue in A) or ionized calcium binding adaptor molecule 1 (Iba1; green in B) staining of superficial laminae of dorsal horn ipsilateral to L5 nerve transection surgery. The first three columns show individual staining of these markers, and the fourth column depicts the merge of all the markers showing coregionalization of CBR2 with perivascular (ED2) and microglial (Iba1) cells.
Fig. 3.
Cannabinoid receptor type 2 (CBR2) staining and localization. Representative confocal images of CBR2 (red), 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; blue) and NeuN (green in A), S100B (green in B) or glial fibrillary acidic protein (GFAP; green in C) staining, and of CBR2 (red), GFAP (gray in D) and ED2/CD163 (ED2; green in D) triple staining of superficial laminae of dorsal horn ipsilateral to L5 nerve transection surgery. The first three columns show individual staining of these markers, and the fourth column depicts the merge of all the markers showing absence of coregionalization of CBR2 with neurons (NeuN) or astrocytes (S100B or GFAP) cells, but showing that astrocytic end-feet are in intimate contact with perivascular cells (ED2) that coregionalize with CBR2.
Fig. 4.
Cannabinoid receptor type 2 (CBR2) staining and localization. Representative confocal images of CBR2 (red), glial fibrillary acidic protein (GFAP; gray), and ED2/CD163 (green) staining of superficial laminae of dorsal horn ipsilateral to L5 nerve transection (L5NT; A) or sham surgery (B). The coregionalization of CBR2 and ED2/CD163 is shown in yellow and was present in almost all of the perivascular cells (ED2/CD163) in L5 nerve transection sections, and to a lesser extent in sham sections. No coregionalization was observed with astrocytes (GFAP) in both groups.
Acute Antinociceptive Effect Study
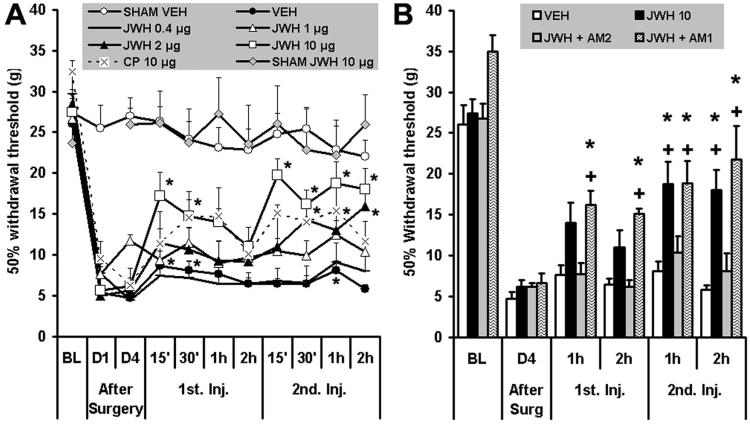
Withdrawal thresholds did not change significantly after sham surgery, and administration of intrathecal vehicle or the CBR2 agonist JWH015 (10 μg, two injections) did not modify this (fig. 5A). This suggests that spinal CBR2 activation does not change baseline nociception. Withdrawal thresholds were reduced significantly (P < 0.05; 28.6 ± 1 g before surgery) at 1 (6.4 ± 0.6 g) and 4 days (5.6 ± 0.4 g) after surgery in all L5NT groups. Intrathecal vehicle induced a slight but significant increase in the withdrawal thresholds 15 and 30 min (8.7 ± 1.8 and 8.1 ± 1.2 g, respectively) after the first injection and 1 h (8.1 ± 1.2 g) after the second injection (fig. 5A). CP55940 (10 μg, two injections) reversed the L5NT-induced hypersensitivity in a significant manner only 1 h after the second injection (18.7 ± 2.8 g). When compared with the L5NT vehicle group, CP55940 (CBR1/2 agonist) induced antinociception 15 min, 30 min, and 1 h after the second injection (32.23 ± 7.9, 29.64 ± 8.9, and 37.8 ± 12.5% maximum possible effect [MPE], respectively). The CBR2 agonist JWH015 induced antinociception in a cumulative dose-dependent manner in L5NT group when compared with after-surgery data and when compared with the L5NT vehicle group (fig. 5A). This is in accord with the inducible CBR2 expression after peripheral nerve injury shown in this study. JWH015 (CBR2 agonist) significantly modified L5NT-induced hypersensitivity after the first intrathecal injection (15 and 30 min) only with the highest dose tested (10 μg, two injections). The second intrathecal injection (2 h after the first one) of 0.4 or 1 μg JWH015 did not modify L5NT-induced hypersensitivity when compared with after-surgery data, but 1 μg JWH015 induced antinociception 15 min after thesecond injection (10.4 ± 1 g, 10.5 ± 5.6% MPE) when compared with the L5NT vehicle group. JWH015 was effective when administered in a dose of 2 μg 30 min and 2 h after the second intrathecal injection when compared with after-surgery data, and 2 h when compared with the L5NT vehicle group (15.9 ± 1.3 g, 49.8 ± 11% MPE). Furthermore, intrathecal 10 μg JWH015 decreased behavioral hypersensitivity starting 15 min after the second injection (19.7 ± 3.7 g, 68.9 ± 19.4% MPE), and this effect lasted at least 2 h (18 ± 2.5 g, 62.7 ± 18% MPE) when compared with both after-surgery data and the L5NT vehicle group. The fact that the first dose of JWH015 (except for the 10-μg group) did not induce any significant effect but the second produced antinociception in a dose-dependent fashion suggests a cumulative effect. The ED50 (95% confidence limits) of JWH015 2 h after the second intrathecal injection (0.8 -20 μg, cumulative dose) was 8.6 (3.6 -20.6) μg.
Fig. 5.
Withdrawal thresholds ipsilateral to sham or L5 nerve transection surgery before (baseline [BL]) and 1 and 4 days (D1 and D4, respectively) after surgery, in L5 nerve transection animals after intrathecal administration of vehicle (VEH), JWH015 (JWH), CP55940 (CP), JWH015 plus AM630 (AM2), or AM281 (AM1), and in sham animals after vehicle (SHAM VEH) or JWH015 (SHAM JWH 10 μg). (A) Withdrawal thresholds to von Frey stimulation ipsilateral to sham or L5 nerve transection surgery before and 1 and 4 days after surgery and 15 min, 30 min, 1 h, and 2 h after first and second injection (1st Inj. and 2nd Inj., respectively) of vehicle (n = 7), CP55940 (10 μg, n = 6), or 0.4, 1, 2, or 10 μg JWH015 (n = 5, 5, 7, and 10, respectively) for L5 nerve transection animals, and JWH015 (10 μg, n = 5) or vehicle (n = 11) in sham animals. Over time, values versus D4 after surgery significantly differ by Friedman test; * P < 0.05 versus D4 after surgery by Friedman test followed by Wilcoxon test. Groups significantly differ by Kruskal-Wallis test; P < 0.05, Kruskal-Wallis test followed by Mann-Whitney U test, was found in L5 nerve transection with CP55940 versus L5 nerve transection with vehicle at 15 min, 30 min, and 1 h after the second injection; L5 nerve transection with 1 μg JWH015 versus L5 nerve transection with vehicle at 15 min after the second injection; L5 nerve transection with 2 μg JWH015 versus L5 nerve transection with vehicle at 2 h after the second injection; L5 nerve transection with 10 μg JWH015 versus L5 nerve transection with vehicle at all times tested after the second injection; sham with vehicle or 10 μg JWH015 versus L5 nerve transection with vehicle at all times tested after surgery. (B) Withdrawal thresholds to von Frey stimulation ipsilateral to L5 nerve transection surgery before and 4 days after surgery (After Surg) and 1 and 2 h after first and second injection of 10 μg JWH015 alone or plus AM630 (10 μg, n = 7) or AM281 (10 μg, n = 7). Groups significantly differ by Kruskal-Wallis test; * P < 0.05 compared with JWH015 plus AM630 group and + P < 0.05 compared with vehicle group by Kruskal-Wallis test followed by Mann-Whitney U test.
The antihypersensitivity effect of intrathecal JWH015 (10 μg, two injections) was prevented by concomitant intrathecal injections of the CBR2 antagonist AM630 (10 μg, two injections; 10.9 ± 1.9 g, 16 ± 8.4% MPE and 8.1 ± 2.2 g, 6.2 ± 11% MPE, 1 and 2 h after second injection) but not by concomitant intrathecal injections of the CBR1 antagonist AM281 (10 μg, two injections; 19 ± 2.8 g, 45 ± 13% MPE and 22 ± 4.1 g, 55 ± 17% MPE, 1 and 2 h after second injection; fig. 5B).
Motor Function and Reflex Testing
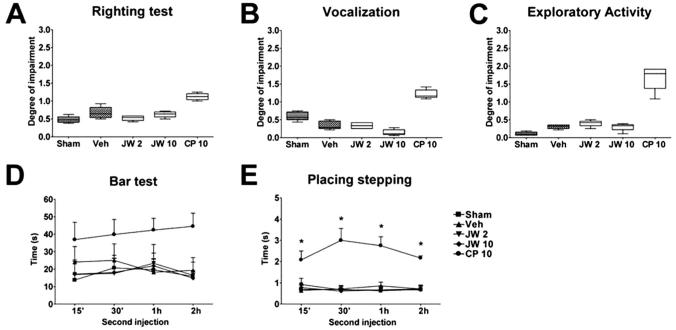
Intrathecal CP55940 (10 μg) did not block the righting reflex (fig. 6A), induce vocalization (fig. 6B), reduce exploratory activity (fig. 6C), or induce catalepsy (fig. 6D) in a significant manner; however, it blocked the placing-stepping reflex at all times tested after the second injection (fig. 6E). In contrast, the CBR2 agonist JWH015 did not affect any of these behavioral measures (figs. 6A-E).
Fig. 6.
Neurologic side effect measures. (A-E) Righting test, vocalization, exploratory activity, bar test, and placing-stepping reflex after the second intrathecal injection of vehicle in sham group (Sham, n = 11), L5 nerve transection with 2 μg JWH015 (JW 2, n = 5), 10 μg JWH015 (JW 10, n = 7), 10 μg CP55940 (CP 10, n = 6), or vehicle (Veh, n = 7). Groups differ in E by repeated-measures two-way analysis of variance, * P < 0.05 compared with L5 nerve transection plus vehicle group repeated-measures two-way analysis of variance followed by the Bonferroni posttest.
JWH015 Effect on ED2/CD163 Expression
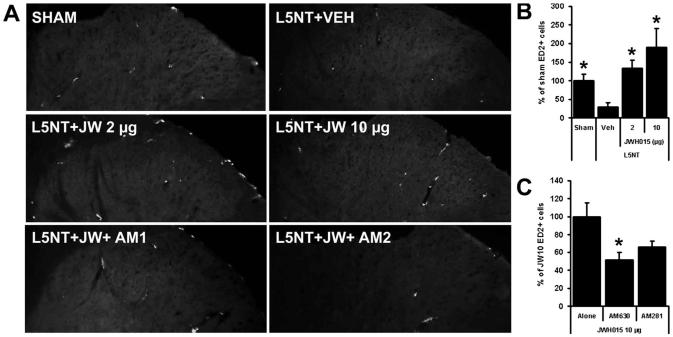
ED2 or CD163 (a glycoprotein member of the scavenger receptor cysteine-rich group B family) functions as a scavenger receptor for hemoglobin-haptoglobin complexes and is constitutively expressed on CNS perivascular cells. ED2/CD163-positive cells and ED2/CD163 staining intensity were significantly reduced in number and intensity in the spinal cord dorsal horn ipsilateral to L5NT (plus vehicle, 3.6 ± 1.6 ED2/CD163+ cells) compared with dorsal horn ipsilateral to sham surgery (plus vehicle, 12.6 ± 2.6 ED2/CD163+ cells; figs. 7A and B). Intrathecal JWH015 (CBR2 agonist) significantly increased the ED2/CD163 expression intensity and the number of ED2/CD163-positive cells ipsilateral to L5NT compared with vehicle controls. The effects of 2 or 10 μg of the CBR2 agonist JWH015 (2 h after the second injection) were similar (16.8 ± 2.7 and 23.9 ± 6.3 ED2/CD163+ cells), paralleling their behavioral effects (figs. 7A and B). Although we did not find any significant difference between JWH015 plus AM281 (CBR1 antagonist, n = 7) versus JWH015 plus AM630 (CBR2 antagonist, n = 5), the effect of JWH015 on ED2/D163 expression is exerted mainly on CBR2 because the increase of ED2/CD163-positive cells (but not the total ED2/CD163 intensity) induced by the highest dose of JWH015 (10 μg) was significantly blocked by AM630 (10 μg, CBR2 antagonist), but not by AM281 (10 μg, CBR1 antagonist; figs. 7A and C).
Fig. 7.
Perivascular cells (ED2/CD163) staining. ED2/CD163 (ED2) staining (A) and percent of sham group number of ED2/CD163-positive cells (B) in superficial dorsal horn ipsilateral to surgery of sham animals plus vehicle (Sham, n = 4) and L5 nerve transection (L5NT) animals plus vehicle (Veh, n = 4), 2 μg JWH015 (JW 2 μg, n = 4), 10 μg JWH015 (JW 10 μg, n = 4), 10 μg JWH015 plus 10 μg AM281 (JW + AM1, n = 3; C), or 10 μg JWH015 plus 10 μg AM630 (JW + AM2, n = 4; C). * P < 0.05 (t test) compared with L5NT plus vehicle group in B and compared with L5NT plus 10 μg JWH015 alone in C.
JWH015 Effect on CR3/CD11b and GFAP Expression
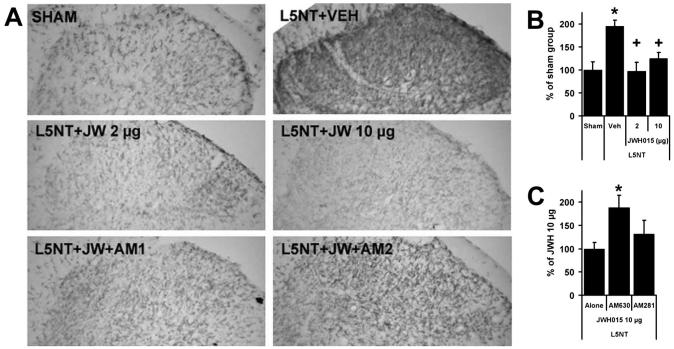
CR3/CD11b (microglia) staining was significantly more intense in the spinal cord dorsal horn ipsilateral to L5NT (plus vehicle) compared with dorsal horn ipsilateral to sham surgery (plus vehicle; figs. 8A and B). CR3/CD11b staining ipsilateral to L5NT was significantly reduced in intrathecal JWH015 (CBR2 agonist)-treated animals compared with vehicle controls. In parallel with JWH015’s effects on behavior, 2 or 10 μg JWH015 (2 h after the second injection) significantly reduced the expression of CR3/CD11b at the same intensity (figs. 8A and B). The reduction of CR3/CD11b expression induced by the highest dose of JWH015 (10 μg) was significantly blocked by AM630 (10 μg, CBR2 antagonist) but not by AM281 (10 μg, CBR1 antagonist; figs. 8A and C). CR3/CD11b staining in sham with intrathecal vehicle, L5NT with intrathecal JWH015, and L5NT with intrathecal JWH015 plus intrathecal AM281 (CBR1 antagonist) groups showed the characteristic morphology of resting microglia (thin and highly ramified processes). L5NT with intrathecal vehicle and L5NT with intrathecal JWH015 plus AM630 (CBR2 antagonist) resulted in microglial cells with larger cell bodies and greatly thickened processes, a characteristic morphology of activated cells (fig. 8A). Four days after L5NT (plus intrathecal vehicle), GFAP (astrocyte marker) staining in the spinal cord dorsal horn was not different from sham with intrathecal vehicle. JWH015 treatment did not modify the intensity of GFAP labeling (data not shown).
Fig. 8.
Microglial (CR3/CD11b) staining. CR3/CD11b staining (A) and percent of sham staining intensity (B) in superficial dorsal horn ipsilateral to surgery of sham animals plus vehicle (Sham, n = 4) and L5 nerve transection (L5NT) animals plus vehicle (Veh, n = 3), 2 μg JWH015 (JW 2 μg, n = 3), 10 μg JWH015 (JW 10 μg, n = 4), 10 μg JWH015 plus 10 μg AM281 (JW + AM1, n = 7; C), or 10 μg JWH015 plus 10 μg AM630 (JW + AM2, n = 5; C). * P < 0.05 (t test) compared with sham plus vehicle group in B and compared with L5NT plus 10 μg JWH015 alone in C. + P < 0.05 (t test) compared with L5NT plus vehicle group.
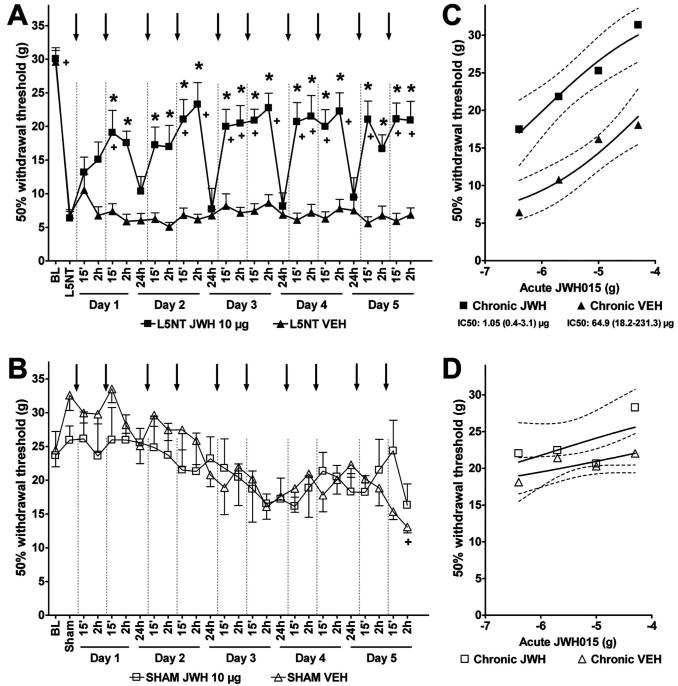
Spinal CBR2 Activation-induced Hypersensitivity Study
To determine whether intrathecal JWH015 (CBR2 agonist) induces hypersensitivity when administered chronically, as reported with other CBR1 agonists,30 we repeated the paradigm described in the acute antinociceptive effect study daily for up to 5 days, using the most effective dose of JWH015 (10 μg, 2 injections/day) observed in the current study. All animals developed hypersensitivity 4 days after L5NT (29.9 ± 1.1 vs. 6.6 ± 0.6 g, before and 4 days after L5NT, respectively), and the chronic intrathecal vehicle treatment (2 injections/day) did not modify this hypersensitivity (fig. 9A). However, intrathecal JWH015 (10 μg, 2 injections/day) reversed L5NT-induced hypersensitivity during the 5 days of treatment compared with postsurgery data and the vehicle group (fig. 9A). This effect was observed after the second injection on day 1 and after the first and second injections on days 2-5. This effect lasted at least 2 h and disappeared 24 h after the second injections at all days tested (fig. 9A). The effectiveness of intrathecal JWH015 was similar at all days tested, suggesting, first, that chronic intrathecal JWH015 administration does not induce hypersensitivity, and second, that a cumulative effect is unlikely when the drug was administered 24 h apart. To determine whether intrathecal JWH015 induces hypersensitivity in nonhypersensitive animals, we repeated the chronic intrathecal JWH015 paradigm described above in sham animals. This was compared with sham animals treated chronically with intrathecal vehicle. Chronic intrathecal JWH015 did not modify the withdrawal thresholds (25.9 ± 2.1 g, after surgery) in sham animals (fig. 9B). However, we observed a slight but significant reduction in the withdrawal threshold after chronic intrathecal vehicle 2 h after the second injection on day 5 treatment (24.5 ± 2.5 vs. 17.3 ± 0.1 g, after surgery and 2 h after second injection on day 5, respectively). Withdrawal thresholds of chronic intrathecal JWH015 in sham surgery animals where significantly different from the L5NT chronic intrathecal vehicle group at all time points tested. However, withdrawal thresholds of the chronic intrathecal vehicle sham surgery group were not significantly different from L5NT chronic intrathecal vehicle 2 h on days 1, 2, and 3, and 15 min and 2 h on days 4 and 5 after the second intrathecal injection of vehicle. These findings suggest a possible transient hypersensitivity induced by multiple injections of the vehicle. Although no statistical differences were found between the two sham groups (JWH015 vs. vehicle), the chronic intrathecal JWH015 group did not show this hypersensitivity. These findings suggest that JWH015 does not induce hypersensitivity in nonsensitized animals when administered chronically.
Fig. 9.
Effects of chronic intrathecal administration of JWH015 and tolerance study. Withdrawal thresholds to von Frey stimulation ipsilateral to L5 nerve transection (L5NT) (A) or sham (B) surgery before (baseline [BL]) and 4 days after surgery (L5NT or Sham), and 15 min and 2 h after the first intrathecal injection and 15 min, 2 h, and 24 h after the second intrathecal injection (arrows and dotted lines) of vehicle (VEH, n = 7 for L5NT and n = 3 for sham) or 10 μg JWH015 (JWH 10 μg, n = 8 for L5NT and n = 5 for sham) during 5 consecutive days. Over time, values versus after-surgery data (L5NT or Sham) significantly differ by Friedman test; + P < 0.05 versus L5NT in A or Sham in B by Friedman test followed by Wilcoxon test. Groups significantly differ by Kruskal-Wallis test in A but not in B;* P < 0.05 compared with vehicle group by Kruskal-Wallis test followed by Mann-Whitney U test. (C and D) Withdrawal thresholds (95% confidence limits, dotted lines) to von Frey stimulation ipsilateral to L5NT (C) or sham (D) surgery 15 min after escalating doses (0.4, 2, 10, and 50 μg) of intrathecal JWH015 administered 24 h after the chronic treatment with 10 μg JWH015 (chronic JWH) or vehicle (chronic VEH). Groups significantly differ by Kruskal-Wallis test in C but not in D; all doses were significantly different between both groups in C (P < 0.05) by Kruskal-Wallis test followed by Mann-Whitney U test. IC50s were significantly different by t test in C (P < 0.05).
Spinal CBR2 Activation-induced Tolerance Study
Because cannabinoid agonists have been shown to induce tolerance after chronic intrathecal administration,30 we further studied whether acute intrathecal administration of the CBR2 agonist JWH015 (escalating doses) remains effective in L5NT and sham animals chronically treated with JWH015 or vehicle. All L5NT animals were hypersensitive before the treatment (6.4 ± 1 and 7.3 ± 0.8 g, 24 h after the day 5 of chronic injections of JWH015 and chronic vehicle, respectively). Escalating doses of intrathecal JWH015 reversed L5NT-induced hypersensitivity in a dose-dependent manner in both chronic JWH015- and chronic vehicle-treated animals. Unexpectedly, acute intrathecal JWH015 was more effective and more potent in chronic JWH015-treated animals than in chronic vehicle-treated ones (fig. 9B). The maximum effect achieved was observed after the highest dose of intrathecal JWH015 in both groups (94.8 ± 3.6% vs. 63.3 ± 14.6% MPE, chronic JWH015 and chronic vehicle groups, respectively; P < 0.05). The ED50 (95% confidence limits) of acute intrathecal JWH015 was 1.05 (0.4 -3.1) and 64.9 (18.2-231.3) μg for the chronic JWH015 and chronic vehicle groups, respectively (P < 0.05). Escalating doses of intrathecal JWH015 did not modify the withdrawal thresholds of the sham surgery group treated chronically with JWH015 or vehicle (22.2 ± 2.2 and 17.3 ± 0.01 g, 24 h after day 5 of chronic treatment of intrathecal JWH015 and intrathecal vehicle, respectively). These findings suggest that spinal CBR2 expression is induced by peripheral nerve injury to induce antinociception. No significant differences were found in all the neurologic tests assessed in both groups after the last JWH015 dose and compared with the L5NT with acute intrathecal vehicle group (data not shown).
Discussion
CBR2 Are Expressed in Spinal Microglial and Perivascular Cells
In the current study, we confirmed that CBR2 expression is enhanced in the dorsal horn ipsilateral to surgery after peripheral nerve injury.19-21 Microglia presumably express CBR2 messenger RNA (mRNA) in the spinal cord under neuropathic pain conditions.20 In accord with this, we show for the first time that CBR2 protein is expressed in microglial and perivascular cells, a subpopulation of microglia, but not in neurons or astrocytes 4 days after peripheral nerve injury. Interestingly, our findings occur 4 days after L5NT, when microglial cells play a key role in the development of pain after peripheral nerve injury.15,18 Others have shown in vivo CBR2 expression in brain microglial cells at early stages (3 days) after stroke and hypoxia-ischemia with no colocalization with astrocytes.31 Also, CBR2-positive staining has been found in human and rat brain perivascular cells and in capillaries surrounded by astrocytes.11,12 In our hands, astrocytes do not express CBR2 4 days after peripheral nerve injury. However, we observed that astrocytic end-feet can be in intimate contact with perivascular cells that do express CBR2, suggesting that CBR2 may indirectly modulate astrocytes.
The constitutive and enhanced expression of CBR2 in immune CNS cells after peripheral nerve injury or other central inflammatory processes suggest that the endocannabinoid system may act in a central immune modulatory role. Because CBR2 are expressed in microglia, especially during inflammation,14 we hypothesize that their activation modulates the proinflammatory phenotype of spinal microglia in neuropathic pain. In contrast with our results, others have failed to show CBR2 expression in spinal microglial cells and have found CBR2 expression mainly in neurons after peripheral nerve injury.21 This discrepancy may be due to nonspecific antibodies, peripheral nerve injury models, or the time after surgery (14 vs. 4 days) used in both studies. A dynamic temporal expression of CBR2 in neuropathic pain conditions may be also a possibility as seen with extracellular signal-regulated kinase expression after peripheral nerve injury.32
Central CBR2 Activation Induces Antinociception
Spinal administration of nonselective CBR agonists (including CBR2 agonists) reduces hypersensitivity in different pain models (including neuropathic pain).25,27 In the current study, we have shown that intrathecal JWH015 induced a dose- and CBR2-dependent antihyper-sensitivity effect after peripheral nerve injury, similar to our previous observations in a postoperative pain model. On the contrary, spinal CBR2 activation does not relieve formalin-induced hypersensitivity25 and does not induce antinociception in sham animals. JWH015 and CP55940 seem to be more effective and potent in the postoperative than in the neuropathic pain model used in the current study.28 This is not surprising because the most difficult type of pain to treat is pain induced by nerve injury.
Spinal CBR2 Activation Modulates Spinal Immune Response
Reactive microglia and astrocytes, as evident by increased expression of Iba1 (microglia marker), CR3/CD11b (microglia marker), and GFAP (astrocyte marker) likely by the increased input and subsequent sensitization of spinal neurons after peripheral nerve injury,33 are involved in the initiation and maintenance of hypersensitivity in neuropathic pain.15-18 In accord with previous studies,17,23 we found widespread CR3/CD11b expression (microglia marker) in the lumbar dorsal horn ipsilateral to L5NT 4 days after surgery. We did not find any robust morphologic change in astrocytic or GFAP (astrocyte marker) expression at this time point, in accord with their later role in neuropathic pain conditions.15,18 We have previously shown an increase in mRNA GFAP and S100B (astrocyte markers) 4 days after L5NT17,34 suggesting that astrocytic activation after L5NT is taking place at this time point in a transcriptional level and that this may not be associated with morphologic or GFAP protein changes.
In accord with the role of microglia in early phases of neuropathic pain, intrathecal JWH015 significantly reduced L5NT-induced CR3/CD11b expression (microglia marker) in a dose- and CBR2-dependent manner in parallel with its behavioral effects. Interestingly, this association was also observed in postoperative pain, suggesting a common pathologic mechanism between both pain models, a possibility that we are currently pursuing. JWH015 and other CBR2 agonists suppress interferon γ-induced CD40 expression, attenuate tumor necrosis factor α and nitric oxide, and regulate migration in microglial cells.35-37 Furthermore, CBR2 activation induces the production of the antiinflammatory factor interleukin-1 receptor antagonist from neurons and glia.38 Interestingly, glial modulation or interleukin-1β and tumor necrosis factor-α inhibition in spinal cord reduces hypersensitivity.16,18 Perivascular cells are CNS antigen-presenting cells39 that play important immune regulatory and surveillance functions.40 These cells are involved in the intimate communication of the CNS with the peripheral immune system in pathologic conditions such as experimental autoimmune encephalomyelitis41 or neuropathic pain conditions.42 ED2/CD163 (CD163) is a specific marker for perivascular cells43 that is expressed on the cell surface or is shed in its soluble form.44 Human macrophages expressing ED2/CD163 are associated with the resolution of inflammation45 and secrete proteins with antiinflammatory properties.46 ED2/CD163 expression is reduced after L5NT, paralleling the L5NT-induced hypersensitivity. Importantly, JWH015 enhanced ED2/CD163 expression in neuropathic animals with the two doses tested at the same intensity, which parallels its behavior effects. These findings suggest that spinal perivascular cells and the decrease of ED2/CD163 may be involved in the development of neuropathic pain. We are currently testing whether L5NT-induced ED2/CD163 down-regulation is related to microglial activation in pain states.
These findings, together with the localization of CBR2, suggest, first, that JWH015 (CBR2 agonist) directly inhibits spinal microglial cells, which would be sufficient to induce a reduction of peripheral nerve injury-induced spinal neuronal activity and behavioral hypersensitivity,28,47,48 and second, that JWH015 affects perivascular cells, inducing the expression of ED2/CD163 and modulating the local immune response. Supporting this hypothesis, other antiinflammatory agents, such as glucocorticoids and interleukin 10, induce the expression of CD163 in human monocytes.49,50
How CBR2 activation is directly inhibiting L5NT-activated microglia remains unknown. We hypothesize that a modulation on extracellular signal-regulated kinase pathway is part of the mechanism of action. Spinal microglial extracellular signal-regulated kinase dephosphorylation reduces neuropathic pain behavior32,47 and microglial CBR2 activation inhibits tumor necrosis factor-α and nitric oxide production by dephosphorylating extracellular signal-regulated kinase.35 We are currently testing this hypothesis. In addition, we hypothesize that ED2/CD163 modulation is part of the mechanism of action by which CBR2 agonists reduce hypersensitivity and the immune response in spinal cord after peripheral nerve injury.
Spinal CBR2 Activation Lacks Side Effects
CBR2 exist in the CNS,9,10 and systemic CBR2 ligands distribute and act in the CNS without inducing overt neurologic side effects.51-53 However, it has recently been shown that brain CBR2 activation may have some neurologic actions.54 We have shown that intrathecal JWH015 did not induce neurologic side effects at anti-hypersensitivity doses in a model of postoperative pain, and we confirm this in a model of neuropathic pain. We cannot exclude the possibility that JWH015 did not induce side effects because of a lack of distribution to supraspinal levels in the CNS or whether the doses used are insufficient to activate higher CNS regions. It is also possible that JWH015 induces cognitive side effects not tested in this study. CP55940 induces its neurologic side effects by its actions on central CBR1,28 which would explain the side effects observed after intrathecal CP55940 in the current study.
Repeated spinal injections of the CBR1 agonist WIN 55,212-2 produced abnormal pain characterized by mechanical and thermal hypersensitivity. Such hypersensitivity seems to manifest as antinociceptive tolerance (decrease in antinociceptive potency).30 Interestingly, we show that chronic spinal CBR2 activation did not induce hypersensitivity or tolerance in sham surgery or L5NT animals using antinociceptive doses of JWH015. Moreover, the potency and efficacy of acute JWH015 increased in L5NT chronic JWH015-treated animals compared with controls. Although the pharmacokinetic properties of cannabinoids administered intrathecally are unknown, the simplest explanation for this observation is the highly lipophilic nature and the long terminal half-life of cannabinoid drugs, such as Δ9-tetrahydrocannabinol (25 h to 5 days), which may favor tissue storage.55,56 The antinociceptive effect achieved by using two doses, but not one, suggests a cumulative effect. However, intrathecal JWH015 was not effective 24 h after its chronic and daily injections, suggesting that the drug is metabolized earlier, making drug accumulation an unlikely reason for its enhanced effectiveness and potency after its chronic administration. Therefore, it seems that other unknown mechanisms may also participate in this intriguing finding.
Conclusions
These data support our hypothesis that CBR2 are expressed in microglia after peripheral nerve injury. We also generated a new hypothesis by finding functional CBR2 in perivascular cells, suggesting a possible role of these cells and ED2/CD163 as immune modulators in neuropathic pain. Our findings suggest that spinal CBR2 activation could be enormously beneficial in pathologies where an excess of microglial activation seems to play a determinant role, including neuropathic, postoperative, or inflammatory pain. Because CBR2 agonists are devoid of some neurologic side effects and antinociceptive tolerance, these data provide the rationale for studying the mechanism of action of these promising CBR2 agonists as potential safer analgesic agents.
Acknowledgments
Supported in part by grant No. DA11276 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 2.Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–12. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–21. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- 4.Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–83. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 5.Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 7.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–96. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–8. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 11.Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci Lett. 2006;396:113–6. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, Tolon RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse. 2004;53:208–13. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- 13.Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49:211–9. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–13. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–30. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 19.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fred-duzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: Possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–8. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–4. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 21.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–45. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005:509–54. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- 23.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 25.Yoon MH, Choi JI. Pharmacologic interaction between cannabinoid and either clonidine or neostigmine in the rat formalin test. Anesthesiology. 2003;99:701–7. doi: 10.1097/00000542-200309000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Sanudo-Pena MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–74. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 27.Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–31. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–94. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- 29.Rahman W, Suzuki R, Webber M, Hunt SP, Dickenson AH. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain. 2006;123:264–74. doi: 10.1016/j.pain.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Gardell LR, Burgess SE, Dogrul A, Ossipov MH, Malan TP, Lai J, Porreca F. Pronociceptive effects of spinal dynorphin promote cannabinoid-induced pain and antinociceptive tolerance. Pain. 2002;98:79–88. doi: 10.1016/s0304-3959(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 31.Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–7. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–59. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 33.De Leo JA, Tawfik ML, LaCroix-Fralish VL. The tetrapartite synapse: Path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Tanga FY, Raghavendra V, Nutile-McMenemy N, Marks A, Deleo JA. Role of astrocytic S100beta in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience. 2006;140:1003–10. doi: 10.1016/j.neuroscience.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 35.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, Rothwell NJ. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci. 2003;23:6470–4. doi: 10.1523/JNEUROSCI.23-16-06470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 40.Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–64. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 41.Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–46. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- 42.Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: Potential relationship to neuropathic pain. Pain. 2002;100:163–70. doi: 10.1016/s0304-3959(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 43.Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989;22:103–6. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- 44.Droste A, Sorg C, Hogger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem Biophys Res Commun. 1999;256:110–3. doi: 10.1006/bbrc.1999.0294. [DOI] [PubMed] [Google Scholar]
- 45.Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the downregulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- 46.Zwadlo-Klarwasser G, Vogts M, Hamann W, Belke K, Baron J, Schmutzler W. Generation and subcellular distribution of histamine in human blood monocytes and monocyte subsets. Inflamm Res. 1998;47:434–9. doi: 10.1007/s000110050357. [DOI] [PubMed] [Google Scholar]
- 47.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owolabi SA, Saab CY. Fractalkine and minocycline alter neuronal activity in the spinal cord dorsal horn. FEBS Lett. 2006;580:4306–10. doi: 10.1016/j.febslet.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 49.Hogger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1883–90. [PubMed] [Google Scholar]
- 50.Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM, Guyre PM. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–21. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 51.Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, Mark L, Pearson MS, Miller W, Shan S, Rabadi L, Rotshteyn Y, Chaffer SM, Turchin PI, Elsemore DA, Toth M, Koetzner L, Whiteside GT. Pharmacological and pharma-cokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–72. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: Pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3:62–7. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 53.Hohmann AG, Tsou K, Walker JM. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J Neurophysiol. 1999;81:575–83. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- 54.Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–36. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 55.Johansson E, Agurell S, Hollister LE, Halldin MM. Prolonged apparent half-life of delta 1-tetrahydrocannabinol in plasma of chronic marijuana users. J Pharm Pharmacol. 1988;40:374–5. doi: 10.1111/j.2042-7158.1988.tb05272.x. [DOI] [PubMed] [Google Scholar]
- 56.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–63. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]