Abstract
Objective:
To compare the responses of patients with spinal cord injury (SCI) in regards to pain and sensory abnormalities to single blinded intravenous (IV) infusions of normal saline, sodium amobarbital, and lidocaine.
Setting:
Inpatient pain unit.
Study Design:
Retrospective chart review.
Methods:
Demographic data, body maps marking pain areas, pain ratings, standardized history and detailed examination were collected on admission in 5 patients with SCI and pain. IV normal saline was followed by either IV sodium amobarbital or lidocaine, respectively, (patients, but not the administering physician, were blinded to the order of the drugs). Spontaneous pain ratings and sensory abnormalities to light touch, pinprick and cold were documented at baseline and immediately after each infusion.
Results:
Sodium amobarbital decreased spontaneous pain by 73% (vs 46% with lidocaine) and normalized sensory abnormalities at or above the level of injury in 3 patients (as compared to just 1 patient with lidocaine), 2 of whom had transitional zone allodynia/hyperalgesia.
Conclusion:
In this small study, the analgesic effect of sodium amobarbital and its ability to modify sensory abnormalities appeared superior to that of lidocaine.
Keywords: Pain, chronic, neuropathic; Sensory deficits; Spinal cord injuries; Analgesia; Sodium amobarbital; Lidocaine
INTRODUCTION
Pain and sensory abnormalities are among the most common consequences resulting from spinal cord injury (SCI) (1,2). Following the inability to walk and bowel or bladder dysfunction, a significant number of individuals with SCI consider chronic pain a very disabling complication (3). Post-SCI pain may arise from multiple and often coexistent pathophysiologic mechanisms (musculoskeletal, neuropathic, visceral, mixed, etc.) (1). Neuropathic pain can be subclassified into pain above or at the level of SCI (ie, pathology at the exiting nerve roots or the spinal cord) and pain below the level of injury (1,4). The latter has been often referred to as “central pain” or “deafferentation pain” (5).
SCI pain and associated sensory abnormalities have been reported to respond fairly poorly to general pharmacological interventions (6). There is some evidence that lidocaine, a local anesthetic known to act by blocking sodium channels, has an effect on neuropathic pain of peripheral (7,8) and central origin (9). On the other hand, intravenous barbiturates have shown some promise in neuropathic pain. The first observation of beneficial effects of barbiturates in central neuropathic pain was made by Tasker et al (10). These authors reported that 82% of a group of patients with post stroke pain syndrome or pain after SCI responded quite well to 50 to 225 mg of IV pentothal (a short-action barbiturate) compared with a 55% response to IV opioids.
Since 1994, we have used IV sodium amobarbital as a diagnostic tool in our inpatient pain unit (Comprehensive Pain Program or CPP, Toronto Western Hospital, Toronto, Ontario). Our experience with the drug has led to several publications summarized in a book chapter (11). In summary, sodium amobarbital, a medium-action barbiturate, has been shown to exert distinctive effects on pain of neuropathic origin (selectively modulating cutaneous allodynia), while it has little affect on nociceptive pain (12–14). It also normalizes sensory abnormalities in patients with unexplainable hypoesthesia (15,16) associated with abnormal brain activation patterns, as we have demonstrated with functional imaging (17).
The current study presents our experience with 5 patients with SCI who were administered IV sodium amobarbital and lidocaine in an inpatient setting as part of investigations to elucidate underlying mechanisms of their pain.
MATERIALS AND METHODS
The charts of 5 patients with SCI and neuropathic pain who were admitted to our inpatient pain unit from 1996 through 2005, were reviewed after permission by the Ethics Board. All patients in this study had intractable pain exceeding 6 months in duration, which was attributed to neuropathic causes based on the specific characteristics and attributes of their pain, as well as the location of their pain. The protocol described elsewhere (12), was established in 1994. It is part of our standard clinical investigations within the context of multidisciplinary assessment and is applied to all inpatients routinely, irrespective of the origin or mechanism of their pain. Written informed consent was obtained from all participants. The consent form stated the following: ‘I understand that I will receive IV administration of any two of the following: lidocaine, normal saline, phentolamine or sodium amobarbital. I understand that I will not be told which drugs I will receive or in which order. I also understand that I may feel no effect at all or that I may become lightheaded, dizzy, develop a stuffed nose, may feel relaxed, happy or sad, and that my pain may increase, decrease or not change at all.’ After all investigations had been finished the patients were told which drugs they received and how they responded.
Data Collection:
Demographic data, body maps where patients marked their pain areas, standardized history, and complete musculoskeletal and neurological examination to determine ASIA impairment scores, were collected on admission. Pain intensity was recorded using an 11-point numerical pain rating scale (where 0 is no pain and 10 worst pain). Cutaneous sensation to touch, pain and cold was tested by a soft brush, a pinprick wheel, and a cold roller, respectively.
Investigational Protocol:
Baseline neuromusculoskeletal examination and pain ratings were obtained before and after intravenous infusion of normal saline, sodium amobarbital, and lidocaine. Sodium amobarbital and lidocaine infusions preceded by normal saline were done on separate days. All patients (but not the clinicians who administered the infusions) were blinded to the actual drugs. Normal saline was infused first (5–10 mL, usually at a rate of 1 mL/min), followed 5 to 10 minutes later by the active drug, ie, sodium amobarbital or lidocaine. Each patient was given a slow bolus of 4 to 7 mg/kg of sodium amobarbital (Eli-Lilly, Indianapolis, IN) intravenously over a period of 7 to 10 minutes (50 mg/mL/min, maximum 500 mg) (Weinstein et al, 1953) (18), or lidocaine 5% up to 5 mg/kg of body weight in a slow IV infusion over 30 minutes in a monitored, post-anesthesia care unit. The data were entered into Microsoft Excel 2003 and the mean and standard deviation of pain scores were calculated.
RESULTS
The study group consisted of 5 patients (3 men and 2 women), with a mean age of 35 years (range 25–45 y) and mean duration of pain 59 months (range: 8–96 mo). Four patients sustained thoracic SCI and 1 patient had cervical SCI. All patients were deemed to suffer from neuropathic pain at or below the level of the injury. Extensive sensory abnormalities ranging from hypoesthesia to hyperalgesia were found in all patients. Details of the clinical problems, pain and sensory findings are reported in Table 1.
Table 1.
Summary of Responses to Analgesic Administration
Normal saline infusion before sodium amobarbital or lidocaine had no effect on pain or sensory abnormalities in all 5 patients. The responses to sodium amobarbital and lidocaine are reported collectively, as well as in each case summary below.
Response to Sodium Amobarbital
Sodium amobarbital (mean dose 253 mg, range 190–350 mg) reduced spontaneous pain by 73% [pain ratings 6.0 (± 1.2) before and 1.6 (± 1.8) (mean ± standard deviation) after infusion]. Sensory abnormalities normalized in 2 patients with transitional zone pain/hyperpathia (cases #4 and #5) and in the upper extremity of a third patient with arm hypoesthesia (case #3).
Response to Lidocaine
Lidocaine (mean dose 297 mg, range 200–450 mg) reduced spontaneous pain by 46% [pain ratings 5.6 (± 1.7) before and 3 (± 2.1) (mean ± standard deviation) after infusion]. Lidocaine infusion resulted in decrease in the transitional zone hyperalgesia and allodynia only in 1 out of the 3 patients whose sensory abnormalities improved substantially with sodium amobarbital (case #4). With both drugs, sensory deficits below the level of the lesion remained unchanged in all patients.
Most common adverse effects reported in all patients with sodium amobarbital were somnolence and lightheadedness, while nausea and dysarthria were reported with lidocaine in 3 out of 5 patients. These effects however, were mild to moderate and of short duration and did not interfere with the evaluations. In addition, during lidocaine infusion, there were no clinically significant abnormalities in heart rate, blood pressure, or ECG findings. Of note, duration of relief after sodium amobarbital or lidocaine ranged from 2 to 6 hours (hence the use of these drugs as diagnostic tools only).
CASE PRESENTATIONS
Case 1.
A 44-year-old woman with T6 ASIA A (7 years post injury) presented with complaints of severe flank pain (at and below the site of the injury). She had no sensation below T6 and no transitional zone sensory abnormality. She failed to respond to normal saline infusion. Pain in the flank was rated at baseline at 6/10 and reduced to 3/10 post sodium amobarbital infusion. Similarly, pain was rated as 7/10 prior to lidocaine infusion and was reduced to 3/10 afterwards. Anesthesia below T6 remained unchanged during both infusions.
Case 2.
A 28-year-old man presented with traumatic T10 ASIA A (8 months post injury) and complaints of severe bilateral leg pains (below the level of the injury). He had no sensation below T10 on the left and T11 on the right, but there was no sensory abnormality at the level of injury. He failed to respond to normal saline. Pain was rated at 6/10 before and 0/10 after sodium amobarbital infusion. Similarly, pain was rated as 5/10 before and 0/10 after lidocaine infusion. Anesthesia as described above remained unchanged during both infusions.
Case 3.
A 45-year-old woman presented with pain complaints in both arms/forearms (at and below the level of injury) 8 years after fracture dislocation of C6-C7 resulting in incomplete SCI with characteristics of Brown-Sequard syndrome. By the time she was seen, she had minor residual effects (fully ambulatory, right drop foot, generalized hyperreflexia, hypoesthesia medial arms and lateral right leg). She was classified as C6 AISA D. She failed to respond to normal saline administration. Her baseline pain was rated 6/10 and was reduced post sodium amobarbital infusion to 3–4/10. (Of note, the burning component in both armpits was completely eliminated.) Sensory hypoesthesia in left arm but not the other extremities was abolished with normalization of sensation. Baseline pain prior to lidocaine infusion was rated 5/10 and remained steady after the infusion together with unchanged sensory abnormalities.
Case 4.
A 25-year-old man presented with T4 AISA A (5 years post injury) and pain at thoracolumbar spine, anterior chest wall and epigastrium (at the level of the injury). Sensory abnormalities consisted of hyperalgesia/allodynia in the transitional zone (T4-T6 area) in a belt-like distribution, while there was complete absence of sensation below the level of T6. He failed to respond to normal saline infusion. Prior to sodium amobarbital infusion, spontaneous pain was rated as 5/10 across the anterior chest wall and 3/10 across the upper abdomen. After sodium amobarbital, spontaneous pain was reduced to 1/10 in both abdomen and anterior chest wall at T4-T6 level. However, both sensory abnormalities and pain remained in the lateral chest wall between the midclavicular and axillary lines. Baseline pain prior to lidocaine infusion was rated 6/10 for the chest wall and 7/10 in the upper abdomen with similar sensory abnormalities at T4-T6 in a belt-like distribution as described above. After lidocaine infusion, all pains were reduced to 3/10 together with resolution of the sensory deficit except in the lateral aspects of the chest wall between the midclavicular and axillary lines (identical sensory changes to those after sodium amobarbital infusion).
Case 5.
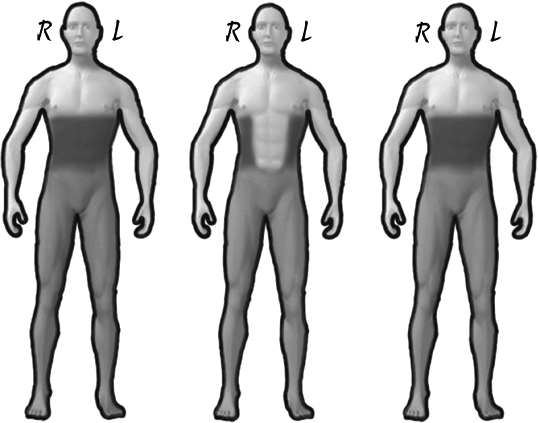
A 26-year-old man with traumatic T4 AISA A (4 years post injury) presented with spontaneous pain in the thoracolumbar spine, anterior chest wall and epigastrium. He had no sensation below T10. However, he displayed marked hyperalgesia and allodynia in a belt-like distribution involving T5-T10 levels (large transitional zone at the level of the injury). He failed to respond to normal saline. Baseline pain was rated 4/10 prior to sodium amobarbital and 0/10 post infusion. Marked reduction of sensory abnormalities was recorded anteriorly, but persisted on the lateral and posterior wall bilaterally. Lidocaine infusion had no effect on pain or sensory abnormalities.
DISCUSSION
The present study examined the effect of intravenous sodium amobarbital and lidocaine on chronic pain and sensory abnormalities in patients with SCI. Administration of both sodium amobarbital and lidocaine significantly reduced the overall spontaneous pain scores by 73% and 46%, respectively, and reduced or normalized sensory abnormalities at the level of the injury. The effect of IV sodium amobarbital, however, seemed superior to that obtained with IV lidocaine both in terms of pain relief and reduction/normalization of sensory abnormalities.
The mechanisms by which sodium amobarbital reduces both pain and sensory abnormalities are unknown. Possibly more than one mechanisms account for the observed effects. Sodium amobarbital has been shown to produce a reversible depression of the central nervous system (CNS), may exert a euphoric effect, and preferentially suppresses polysynaptic responses both at the level of the spinal cord and subcortical and cortical levels (19). On the peripheral nervous system, sodium amobarbital selectively depresses transmission through autonomic ganglia and reduction of choline esters nicotinic excitation (19). The inhibitory effects of sodium amobarbital have been reported to occur at the gamma-aminobutyric acid (GABA) sites, and at the NMDA (n-methyl d-aspartate) receptor as a non-competitive receptor antagonist (20). In general, sodium amobarbital enhances GABA-A inhibition in multiple peripheral and central nervous system sites and also exerts noncompetitive antagonistic effects on AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, as well as kainate and NMDA receptors (13). It is possible that the noncompetitive NMDA-receptor antagonistic action of sodium amobarbital may be responsible for the substantial alteration of cutaneous (centrally mediated) hyperesthesia (20).
The temporary reversal of transitional zone hypersensitivity points to normalization of dynamic (non structural) abnormalities of the CNS and particularly central sensitization, while irreversible hyperesthesia is likely due to local nociceptor sensitization. The normalization of arm hypoesthesia in case #3 again points to temporary reversal of “dynamic” sensory deficit” (12) in contrast to the anatomically based anesthesia below the injury level, which did not change. However, it is unclear what the mechanism is behind normalization of dynamic hypoesthesia. Of note, similar findings in regards to pain have been seen in other studies involving short-acting barbiturates such as sodium thiopental in reducing centrally mediated pain (21).
Unfortunately, the effects of IV sodium amobarbital can not be reproduced by oral administration of the drug resulting in similar serum concentrations. This analgesic effect of sodium amobarbital is attributed to the speed with which the drug crosses the blood brain barrier when administered IV (11). Therefore, at present, the drug is used only as a diagnostic tool to elucidate the functional (dynamic) vs structural mechanisms of sensory abnormalities and pain.
CONCLUSION
In summary, our study demonstrates that the effects of sodium amobarbital infusion in patients with neuropathic pain secondary to SCI seem to be superior to those of IV lidocaine both in terms of pain relief and improvement of sensory abnormalities. However, our study has significant limitations as it was of small size, retrospective in nature and the patients were single blinded. These limitations arise from the fact that the study is “pragmatic,” conducted in the context of regular care within an acute hospital, and not experimental (at which time double blinding and other changes in the protocol could allow for greater scientific accuracy). If larger well designed studies can duplicate our findings, this may lead to future development of novel pharmacological targets.
Figure 1. Effects of infusions on patient #5. Transitional zone hypersensitivity (dark shaded area) at baseline (left), after sodium amobarbital (center) and after lidocaine infusion (right). Anesthesia below level of injury (light-shaded area) remained unchanged.
REFERENCES
- Siddall PJ, Loeser JD.Pain following spinal cord injury Spinal Cord. 200139 (2) 63–73. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, Wolfe DL, Hsieh JT, Hayes KC, Durham CE.Quantitative sensory testing in patients with incomplete spinal cord injury Arch Phys Med Rehabil. 199980 (10) 1258–1263. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Felipe-Cuervo E, Broton JG, Duncan RC, Yezierski RP.Perceived difficulty in dealing with consequences of spinal cord injury Arch Phys Med Rehabil. 199980 (5) 580–586. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Cousins MJ.Spinal pain mechanisms Spine. 199722 (1) 98–104. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, Cousins MJ.Classification of pain following spinal cord injury Spinal Cord. 199735 (2) 69–75. [DOI] [PubMed] [Google Scholar]
- MacFarlane BV, Wright A, O'Callaghan J, Benson HA.Chronic neuropathic pain Pharmacol Ther. 199775 (1) 1–19. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Shafer SL, Yaksh TL.Prolonged alleviation of tactile allodynia by intravenous lidocaine in neuropathic rats Anesthesiology. 199583 (4) 775–785. [DOI] [PubMed] [Google Scholar]
- Jaffe RA, Rowe MA.Subanesthetic concentrations of lidocaine selectively inhibit a nociceptive response in the isolated rat spinal cord Pain. 199560 (2) 167–174. [DOI] [PubMed] [Google Scholar]
- Attal N, Gaude V, Brasseur L, et al. Intravenous lidocaine in central pain: a double-blind, placebo-controlled, psychophysical study Neurology. 200854 (3) 564–574. [DOI] [PubMed] [Google Scholar]
- Tasker RR, de Carvalho G, Dostrovsky JO. The history of central pains syndromes, with observations concerning pathophysiology and treatement. In: Casey KL, editor. Pain and Central Nervous System Disease: The Central Pain Syndromes. New York: Raven Press; 1991. pp. 31–58. [Google Scholar]
- Mailis A, Nicholson K.The use of sodium amytal in functional and other disorders Physical Medicine & Rehabilitation: State of the Art Reviews. 200216 (1) 131–146. [Google Scholar]
- Mailis A, Plapler P, Ashby P, Shoichet R, Roe S.Effect of intravenous sodium amytal on cutaneous limb temperatures and sympathetic skin responses in normal subjects and pain patients with and without complex regional pain syndromes (type I and II). I Pain. 199770 (1) 59–68. [DOI] [PubMed] [Google Scholar]
- Mailis A, Amani N, Umana M, Basur R, Roe S.Effect of intravenous sodium amytal on cutaneous sensory abnormalities, spontaneous pain and algometric pain pressure thresholds in neuropathic pain patients: a placebo-controlled study. II Pain. 199770 (1) 69–81. [DOI] [PubMed] [Google Scholar]
- Mellegers MA, Furlan AD, Mailis A.Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature Clin J Pain. 200117 (4) 284–295. [DOI] [PubMed] [Google Scholar]
- Mailis A, Papagapiou M, Umana M, Cohodarevic T, Nowak J, Nicholson K.Unexplainable nondermatomal somatosensory deficits in patients with chronic nonmalignant pain in the context of litigation/compensation: a role for involvement of central factors J Rheumatol. 200128 (6) 1385–1393. [PubMed] [Google Scholar]
- Sa DS, Mailis-Gagnon A, Nicholson K, Lang AE.Posttraumatic painful torticollis Mov Disord. 200318 (12) 1482–1491. [DOI] [PubMed] [Google Scholar]
- Mailis-Gagnon A, Giannoylis I, Downar J, et al. Altered central somatosensory processing in chronic pain patients with “hysterical” anesthesia Neurology. 200360 (9) 1501–1507. [DOI] [PubMed] [Google Scholar]
- Weinstein EA, Kahn RL, Sugarman LA, et al. The diagnostic use of amobarbital sodium (“amytal sodium”) in brain disease. Am J Psychiatry. 1953;109:889–894. doi: 10.1176/ajp.109.12.889. [DOI] [PubMed] [Google Scholar]
- Downing OA.The effects of procaine, amylobarbitone on drug induced changes in the surface potentials of an isolated sympathetic ganglion Br J Pharmacol. 197245 (1) 159P–160P. [PMC free article] [PubMed] [Google Scholar]
- Taverna FA, Cameron BR, Hampson DL, Wang LY, MacDonald JF.Sensitivity of AMPA receptors to pentobarbital Eur J Pharmacol. 1994267 (3) R3–R5. [DOI] [PubMed] [Google Scholar]
- Wajima Z, Shitara T, Inoue T, Ogawa R.Severe lightning pain after subarachnoid block in a patient with neuropathic pain of central origin: which drug is best to treat the pain Clin J Pain. 200016 (3) 265–269. [DOI] [PubMed] [Google Scholar]