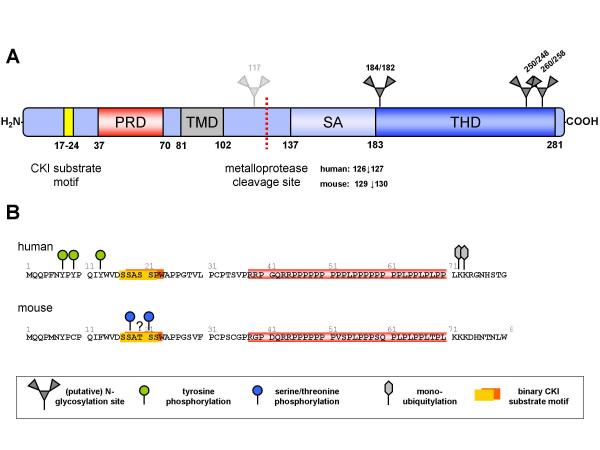
Figure 2.
FasL structure and potential sites of posttranslational modifications. Schematic representation of FasL structure (A). FasL is a type II membrane protein characterised by its unique cytosolic tail containing a binary casein kinase I (CKI) substrate motif (yellow) and a proline-rich domain (PRD). C-terminal to its transmembrane region (TM), a stretch comprising aa 137 to 183 in human FasL was shown to be essential for trimerisation and self-assembly of FasL (SA). The C-terminal moiety is characterised by its homology to other TNF ligand ectodomains (THD). Cleavage sites for metalloprotease-mediated ectodomain release have been mapped for human FasL in a human and murine cellular environment as indicated. FasL contains three putative glycosylation sites (N184, N250, N260), whereas murine FasL contains four (N117, N182, N248, N258). Previously described covalent posttranslational modifications of the human FasL and murine FasL cytosolic tails (B). As detailed in our review, murine FasL might be subject to serine/threonine phosphorylation of the CKI substrate region, but such sites have not been exactly mapped to individual residues within this motif.