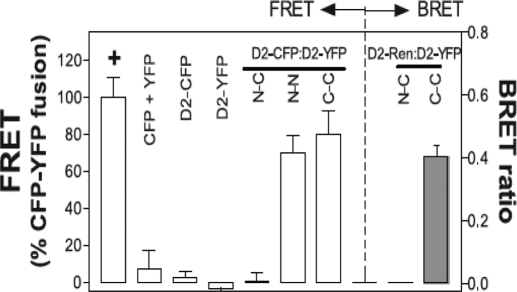
Figure 3.
Deiodinases are dimers. FRET measures the transfer of energy from an excited CFP-tagged molecule to a YFP-tagged acceptor molecule in close proximity. The FRET between D2-CFP and D2-YFP fusion proteins in transfected cells is shown. The location of the CFP and YFP chromophores relative to the D2 protein is indicated by N (amino) or C (carboxyl), respectively, and results are expressed as a percentage of the FRET of a positive control YFP-CFP fusion protein indicated by a plus symbol. CFP + YFP is a negative control for both proteins expressed alone. Notably, D2 with an N-terminal fusion of CFP has no FRET with D2 a C-terminal fusion of YFP, whereas FRET is observed between an N-terminal fusion of CFP to D2 and a N-terminal fusion of YFP to D2, or between a C-terminal fusion of CFP to D2 and C-terminal fusion of YFP to D2 (left panel, last 3 columns). For the bioluminescence resonance energy transfer studies (right panel), YFP fused to the N or C terminus of D2 and Renilla luciferase fused to the C terminus of D2 were expressed in transfected cells. Luminesence produced by the luciferase molecule can then excite a YFP molecule in close proximity, and the resulting YFP emission is measured. YFP, Yellow fluorescent protein; CFP, cyan fluorescent protein. [Reprinted with permission from Sagar et al.: Mol Cell Biol 27:4774–4783, 2007 (44). ©The American Society for Microbiology.]