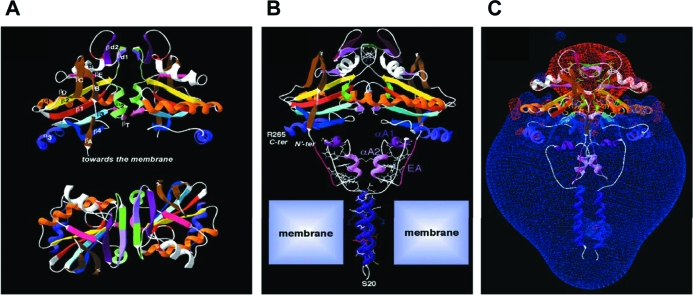
Figure 4.
Globular interfaces mediate D2 dimerization and are critical for catalytic activity. A, Two orthogonal views of the modeled D2 dimer on the template of the crystal structure of human TRX dimer. At the top, the twofold axis is vertical, and at the bottom, it is perpendicular to the figure. Secondary structures are colored. The putative structure of the iduronidase-like active site insertion has been modeled as a ββ secondary structure (βd1 and βd2) lying between β2 and αB. βd1 is green/light purple, βd2 is dark purple, and, at the bottom of the dimer, the two symmetrical small βT in pink are the counterparts of the canonical TRX pairing. B, 3D model of the D2–D2 homodimer. N′ter and Cter indicate the N terminus of the TRX fold head domain and the C terminus of D2, respectively. A single large cavity is created upon D2 dimerization at the level of the active site (Sec133). C, Visualization of the Russian-doll-shaped electrostatic field around the D2 dimer (the −1.8-kT/e gradient limit is red and the +1.8-kT/e gradient limit is blue). [Reprinted with permission from Sagar et al.: Mol Cell Biol 27:4774–4783, 2007 (44). ©The American Society for Microbiology.]