Abstract
We report the synthesis and in vitro antimalarial activities of more than 50 7-chloro-4-aminoquinolyl-derived sulfonamides 3-8 and 11-26, ureas 19-22, thioureas 23-26, and amides 27-54. Many of the CQ analogues prepared for this study showed submicromolar antimalarial activity versus HB3 (chloroquine sensitive) and Dd2 (chloroquine resistant strains of P. falciparum) and low resistance indices were obtained in most cases. Systematic variation of the side chain length and introduction of fluorinated aliphatic and aromatic termini revealed promising leads that overcome CQ resistance. In particular, sulfonamide 3 exhibiting a short side chain with a terminal dansyl moiety combined high antiplasmodial potency with a low resistance index and showed IC50‘s of 17.5 nM and 22.7 nM against HB3 and Dd2 parasites.
Introduction
Malaria is one of the world’s most widespread infectious diseases afflicting approximately 300 million people annually. About 2 million people, mostly young children in tropical and subtropical regions, die of malaria every year which corresponds to more than 5,000 casualties per day. Malaria in humans is caused by protozoan parasites of the genus Plasmodium and is transmitted by the Anopheles mosquito. Although effective antimalarial agents have been known for a long time, the alarming spread of drug resistant strains of P. falciparum, which is the most lethal parasite species, underscores the urgency and continuous need for the discovery of new therapeutics. 7-Chloro-4-aminoquinoline derivatives including chloroquine (CQ), sontoquine, and amodiaquine are among the most potent antimalarial drugs reported to date,1-3 and new agents with improved activity against CQ resistant (CQR) strains have been introduced via synthetic modifications of the CQ side chain.4-7
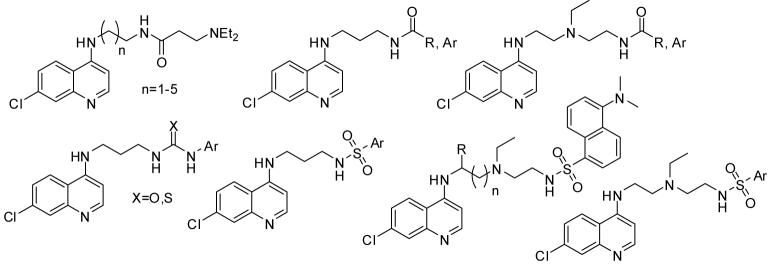
Chloroquine and other aminoquinolines are commonly believed to inhibit the formation of crystalline hemozoin from free ferriprotoporphyrin IX (FPIX) which is toxic to the parasite and generated during proteolysis of host hemoglobin in the acidic food vacuole in the infected red blood cell.8 The interaction between the aminoquinoline ring of CQ and FPIX thus interferes with the detoxification mechanism of the parasite and ultimately impedes proliferation.9-15 The mechanism of CQ resistance is not fully understood but it is known that resistant strains accumulate reduced amounts of CQ in the digestive food vacuole (DV) relative to their CQ sensitive (CQS) counterparts. This has been attributed to mutations in the DV membrane protein Pfcrt (P. falciparum chloroquine resistance transporter) which might result in reduced accumulation of CQ in CQR strains.16-18 Since the trapping of high concentrations of heme-targeted antimalarial drugs in the DV is essential, many efforts have been directed to overcome the resistance mechanism and the drug recognition by Pfcrt via modification of the CQ side chain. Noteworthy, CQR reversal agents19,20 and new treatments21 such as artemisinin and related 1,2,4-trioxolanes have been developed.22-27 A remaining drawback of 1,2,4-trioxolanes is that they are generally less affordable in third world countries while resistance to some organoperoxides has already emerged.28-30 By contrast, 4-aminoquinolines are typically less expensive and have good activity-toxicity profiles.31 The search for new CQ analogues that are equally effective against CQS and CQR strains has therefore received increasing attention during recent years. Various studies have revealed that structural changes of the 7-chloroquinoline ring in CQ reduces the antimalarial activity,14,32 whereas modifications of the CQ side chain appears to be more promising. We have prepared several 4-amino-7-chloroquinolyl-derived amides, sulfonamides, ureas and thioureas and discuss the antimalarial activity of these new compounds herein, Figure 1.
Figure 1.
General structures of tested 4-amino-7-chloroquinolyl-derived amides, sulfonamides, ureas and thioureas.
Results and discussion
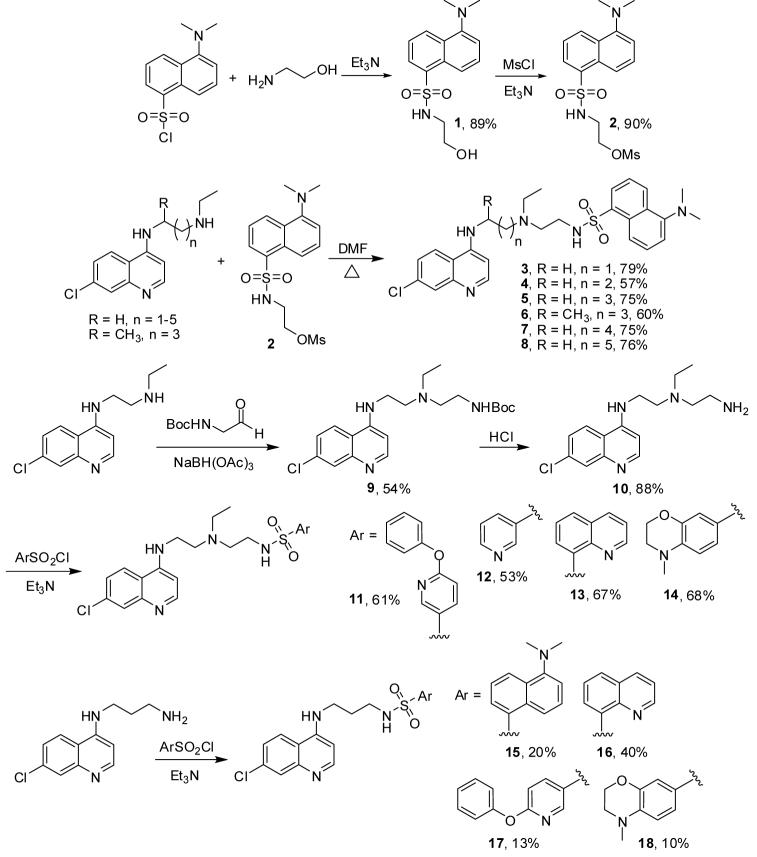
Sulfonamides including the protease inhibitor and antiretroviral fosamprenavir, the nonsteroidal anti-inflammatory drug celecoxib, and sumatriptan, which has been used to treat migraine headaches, have found widespread use as pharmaceuticals. Among the few examples of antimalarial sulfonamides reported to date, some exhibit remarkable potency.33-36 We therefore decided to prepare CQ-derived sulfonamides 3-8 and 11-18, Scheme 1. Following a literature procedure, we synthesized 1 in 89% yield from dansyl chloride and aminoethanol.37 Treatment of 1 with methanesulfonyl chloride gave the corresponding mesylate 2 in 90% yield which allowed formation of sulfonamides 3-8 from a series of N-(7-chloro-4-quinolyl)-1,n-diaminoalkanes. Reductive amination of N-(7-chloro-4-quinolyl)-N’-ethyl-1,2-diaminoethane in the presence of N-t-Boc-glycinal gave chloroquinoline 9 in 54% yield. Deprotection furnished 10 which was then converted to arylsulfonamides 11-14 in good yields. Using a similar approach, 15-18 were prepared from N-(7-chloro-4-quinolyl)-N’-propyl-1,3-diaminoethane and an arylsulfonyl chloride in a single step.
Scheme 1.
Synthesis of sulfonamides 3-8, 11-14 and 15-18.
The antiplasmodial activity of these compounds was measured versus a CQS (HB3) and a CQR (Dd2) strain using a standardized, inexpensive assay based on SYBR Green I intercalation.38-40 The IC50 values were calculated from experiments carried out in triplicate and compared to CQ (Table 1). Sulfonamides 3-8 represent a series of CQ analogues with systematically varied side chain length and a dansyl unit attached to the diethylamino terminus. All compounds within this series showed antimalarial activity against both strains tested and a low resistance index (RI). The RI provides a quantitative measurement of the antiplasmodial activity against CQR strains relative to that against CQS strains and reveals promising drug discovery leads. We found that the RI for 3-8 range from 0.5 to 3.6 whereas the resistance index of CQ was determined as 11.8. Most remarkable in this series is that the short chain 7-chloro-4-aminoquinolyl sulfonamide 3 proved significantly more potent against the resistant strain Dd2 relative to CQ. Compound 3 gave IC50‘s of 17.5 and 22.7 nM against HB3 and Dd2, respectively. It thus retained its potency even when tested against a CQR strain. An increase in the chain length proved detrimental to the antimalarial activity. However, a maximum of the IC50‘s against the CQS and the CQR strains was obtained for compound 4 exhibiting three methylene units between the 4-aminoquinoline moiety and the tertiary amino function. Krogstad previously reported a somewhat similar trend for the antimalarial potency of CQ derivatives with varying side chain length against Indochina I, a CQR strain, but not against Haiti 135, a CQS strain.41 Interestingly, comparison of compounds 5 and 6 shows that introduction of a methyl group, which perfectly mimics the side chain of CQ, reduces the activity against both strains tested. Exchange of the 6-dimethylaminonaphthyl group in 3 by other aromatic groups furnished sulfonamides 11-14. These compounds gave similar RI values, ranging from 1.4 to 4.1, but showed lower antimalarial activity than 3, which indicates the significance of the terminal dansyl group. The basic tertiary amino function in the side chain is commonly believed to be crucial for the accumulation of the drug within the acidic food DV. The IC50‘s of sulfonamides 15-18 therefore increased into the micromolar range.
Table 1.
Antiplasmodial activity of CQ-derived sulfonamides, ureas and thioureas against HB3 and Dd2
| Strain/IC50 (nM)a | |||
|---|---|---|---|
| Compound | HB3 | Dd2 | RIb |
| CQ | 10 | 127 | 12.7 |
| 3 | 18 | 23 | 1.3 |
| 4 | 115 | 411 | 3.6 |
| 5 | 82 | 125 | 1.5 |
| 6 | 220 | 260 | 1.2 |
| 7 | 70 | 115 | 1.7 |
| 8 | 272 | 147 | 0.5 |
| 11 | 140 | 274 | 2.0 |
| 12 | 124 | 512 | 4.1 |
| 13 | 310 | 443 | 1.4 |
| 14 | 171 | 286 | 1.7 |
| 15 | 754 | 966 | 1.3 |
| 16 | 453 | 607 | 1.3 |
| 17 | >1000 | >1000 | - |
| 18 | 960 | >1000 | - |
| 19 | 316 | 596 | 1.9 |
| 20 | 281 | >1000 | - |
| 21 | 436 | 674 | 1.6 |
| 22 | 365 | 539 | 1.5 |
| 23 | 229 | 353 | 1.5 |
| 24 | 208 | 801 | 3.9 |
| 25 | 272 | 482 | 1.8 |
| 26 | 291 | 606 | 2.1 |
IC50 values were obtained from an average of two separate determinations each performed in triplicate.
Resistance Index (CQR-IC50/CQS-IC50).
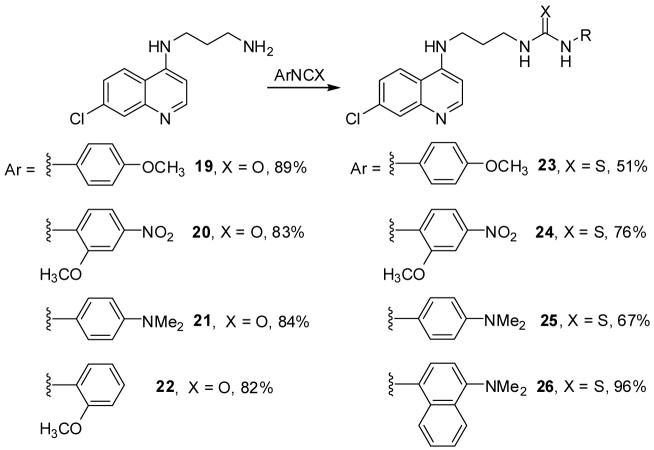
4-Amino-7-chloroquinolyl-derived ureas and thioureas 19-26 were prepared in good to high yields from N-(7-chloro-4-quinolyl)-1,3-diamine and the corresponding isocyanate and isothiocyanate, respectively, as shown in Scheme 2. Almost all compounds studied showed submicromolar antiplasmodial activity (Table 1). These results compare favorably with the majority of previously reported chloroquine-derived ureas and thioureas.42,43 However, Chibale et al. found that urea analogues of ferrochloroquine afford superior antiplasmodial activity against a sensitive (D10) and a resistant (K1) strain compared to CQ.44 In analogy to the sulfonamides discussed above, the low RI values of 19-26 are impressive and suggest that incorporation of a rigid urea or thiourea group into the side chain provides new leads that overcome drug resistance to heme-targeted antimalarials.
Scheme 2.
Preparation of ureas and thioureas 19-26.
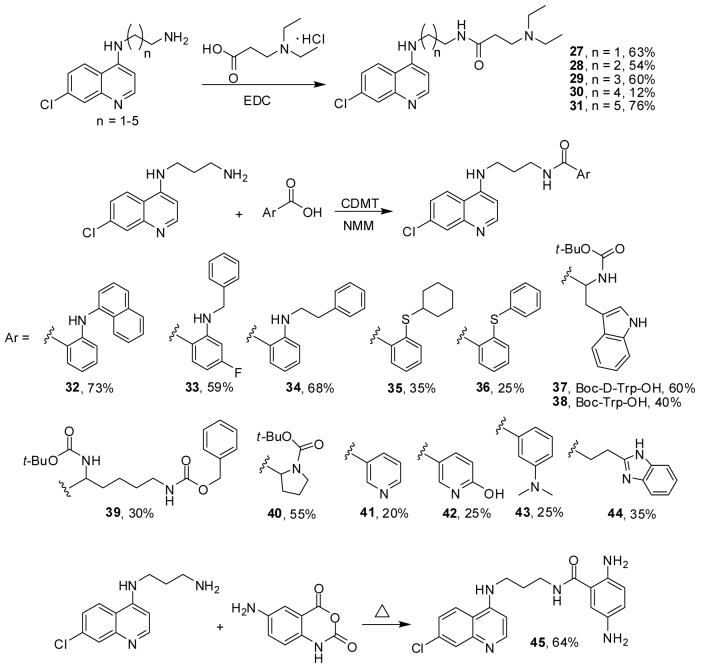
The incorporation of amide functionalities into the side chain of primaquine,45 amodiaquine46,47 and chloroquine48-52 has led to a remarkable range of promising antimalarial agents. For comparison with the sulfonamides and ureas discussed above, we prepared chloroquine-derived amides 27-45, Scheme 3. Coupling of N-(7-chloro-4-quinolyl)-1,n-diaminoalkanes of varying chain length and N,N-diethylamino-3-propionic acid in the presence of 1-[3-(dimethylaminopropyl]-3-ethylcarbodiimide (EDC) gave 27-31. By contrast, we found that superior results in the syntheses of 32-44 are obtained when 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) is used as coupling agent. The anthranilic acids and 2-alkylthio- and 2-arylthiobenzoic acids used in the final coupling step towards 32-36 were prepared as reported previously.53-55 Chloroquine-derived amide 45 was directly prepared from N-(7-chloro-4-quinolyl)-1,3-diamine and 5-aminoisatoic anhydride in 64% yield.
Scheme 3.
Synthesis of amides 27-45.
The amide series 27-31 shows high activity against HB3 (IC50‘s range from 16.3 to 31.5) but generally less potency against the chloroquine resistant strain Dd2 (Table 2). The IC50‘s against HB3 do not vary substantially with the chain length. However, comparison of the IC50‘s obtained with Dd2 reveals a maximum for 29 which has 4 methylene groups between the 4-aminoquinolyl unit and the amido nitrogen. Apparently, alteration of the chain length again provides an effective tool in the search of new drug candidates that retain their antiplasmodial potency against CQR strains. All other amides prepared proved less effective against both HB3 and Dd2 but we noticed that the 2-benzylamino-4-fluorobenzoyl derivative 33 was significantly more active against Dd2 than HB3. The higher activity against the CQR strain was even more surprising because this was not the case for its defluorinated analogue 34.
Table 2.
Antiplasmodial activity of CQ-derived amides against HB3 and Dd2
| Strain/IC50 (nM)a | |||
|---|---|---|---|
| Compound # | HB3 | Dd2 | RIb |
| CQ | 10 | 127 | 12.7 |
| 27 | 32 | 760 | 24.1 |
| 28 | 23 | 559 | 24.4 |
| 29 | 29 | >1000 | - |
| 30 | 16 | 672 | 41.2 |
| 31 | 28 | 385 | 12.9 |
| 32 | >1000 | >1000 | - |
| 33 | >1000 | 305 | - |
| 34 | >1000 | >1000 | - |
| 35 | >1000 | >1000 | - |
| 36 | >1000 | >1000 | - |
| 37 | 500 | 764 | 1.5 |
| 38 | 479 | >1000 | - |
| 39 | 902 | >1000 | - |
| 40 | >1000 | >1000 | - |
| 41 | >1000 | >1000 | - |
| 42 | >1000 | >1000 | - |
| 43 | 449 | 792 | 1.8 |
| 44 | 756 | >1000 | - |
| 45 | 179 | 651 | 3.6 |
IC50 values were obtained from an average of two separate determinations each in triplicate.
Resistance Index (CQR-IC50/CQS-IC50).
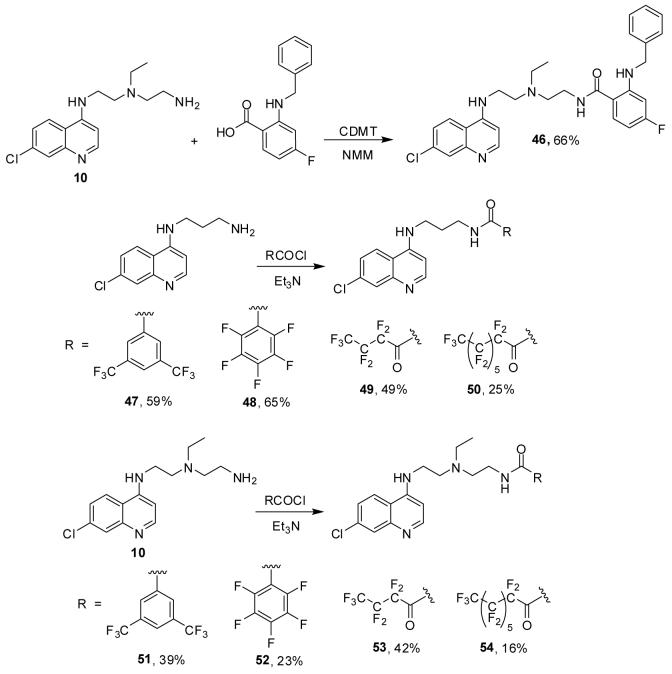
Based on the relatively high activity of 33 against Dd2, we decided to synthesize several additional fluorinated CQ amides (Scheme 4). While amide 46 was prepared via CDMT mediated coupling of 10 with the corresponding benzoic acid derivative, all other amides were obtained using acyl chlorides. We were pleased to find that these fluorinated CQ amides show improved activity compared to 32-45 (Table 3). More importantly, fluoro amides 46-54 have excellent RI values ranging from 1.2 to 3.1. This compares favorably with the high RI’s determined for amides 27-31, and it underscores that incorporation of fluorinated terminal groups into the CQ side chain can possibly provide a means to circumvent the CQR mechanism.
Scheme 4.
Synthesis of amides 46-54.
Table 3.
Antiplasmodial activity of fluorinated CQ-derived amides 46-54
| Strain/IC50 (nM)a | |||
|---|---|---|---|
| Compound | HB3 | Dd2 | RIb |
| CQ | 10 | 127 | 12.7 |
| 46 | 80 | 199 | 2.5 |
| 47 | 145 | 236 | 1.6 |
| 48 | 276 | 510 | 1.9 |
| 49 | 234 | 277 | 1.2 |
| 50 | 166 | 197 | 1.2 |
| 51 | 97 | 137 | 1.4 |
| 52 | 118 | 314 | 2.7 |
| 53 | 180 | 556 | 3.1 |
| 54 | 72 | 87 | 1.2 |
IC50 values are an average of two separate determinations each done in triplicate.
Resistance Index (IC50 CQR)/(IC50 CQS)
Conclusion
More than 50 antiplasmodial 7-chloro-4-aminoquinolyl-derived sulfonamides, ureas, thioureas and amides have been synthesized and tested against CQR and CQS P. falciparum. Many of the CQ analogues prepared for this study showed submicromolar antimalarial activity versus HB3 and Dd2 and low resistance indices. The effects of side chain length, the presence of urea, thiourea, amide and sulfonamide functionalites, and the introduction of fluorinated aliphatic and aromatic termini on the potency against CQS and CQR strains of P. falciparum was investigated. Although none of the quinolyl antimalarials tested was as active as CQ against HB3, more importantly, sulfonamide 3 showed improved activity against the CQR strain Dd2. The results revealed interesting SAR principles leading to promising new directions for the design of antimalarials that address CQ resistance. In particular, sulfonamide 3 exhibiting a short side chain with a terminal dansyl moiety proved significantly more potent against the resistant strain Dd2 than CQ, and incorporation of fluorinated termini into the CQ side chain gave desirable RI indices.
Experimental
Cell Culture and Antimalarial Activity Measurements
Drug activities were assessed and IC50 were quantified essentially as described previously.38-40 The aminoquinolines were diluted using complete media under sterile conditions and plated in a 96 well plate format. Sorbitol synchronized cultures were utilized with >95% of the parasites at the ring stage. Cultures were diluted to give a working stock of 0.5% parasitemia and 2% hematocrit (final hematocrit 1% & 0.5% Parasitemia). The plates were incubated for 72 h at 37 °C. After 72 h, 50 μL of 10x SYBR green I dye was added to each well, and the plate was incubated for 1 h at 37 °C. Fluorescence was measured at 530 nm (490 nm excitation) using a spectra geminiEM plate reader. Data analysis was performed using Sigma plot 9.0 software after downloading data in Excel format. For each assay, each drug dilution was analyzed in triplicate, and the results from at least two separate assays were averaged in each case (S.D. < 10% in each case). All drugs were tested against one chloroquine sensitive, and one chloroquine resistant strain of P. falciparum (HB3 and Dd2, respectively). Based on NMR spectroscopic and HPLC chromatographic analyses, all compounds were at least of 98% purity.
Synthesis
General
All reagents and solvents were commercially available and used without further purification. Flash chromatography was performed on Kieselgel 60, particle size 0.032-0.063 mm. NMR spectra were obtained on a 300 MHz (1H-NMR) and 75 MHz (13C-NMR) Varian FT-NMR spectrometer using CDCl3 as solvent unless indicated otherwise. N-(7-Chloro-4-quinolyl)-1,n-diaminoalkanes and N-(7-chloro-4-quinolyl)-N’-ethyl-1,n-diaminoalkane derivatives were synthesized following a procedure reported in the literature.56
2-(5-Dimethylaminonaphthalene-1-sulfonamido)ethyl methanesulfonate, 2
To a solution of 5-dimethylamino-N-(2-hydroxyethyl)naphthalene-1-sulfonamide, 1 (1.5 g, 5.1 mmol) and Et3N (1.07 mL, 7.64 mmol) in anhydrous CH2Cl2, methanesulfonyl chloride (0.44 mL, 5.61 mmol) was added at room temperature and stirred for 1 hour. After addition of water, the reaction mixture was extracted with CH2Cl2, dried over anhydrous Na2SO4 and concentrated in vacuo. Flash chromatography using EtOAc:hexanes (1:4 v/v) as mobile phase and gradually changing the ratio of EtOAc:hexanes to 1:1.7 (v/v) afforded 1.71 g (4.6 mmol, 90% yield) of a light yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 2.86 (s, 3H), 2.89 (s, 6H), 3.27 (m, 2H), 4.17 (t, J = 6.0 Hz, 2H), 5.11 (t, J = 6.0 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 7.50-7.64 (m, 2H), 8.22-8.28 (m, 2H), 8.57 (d, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 37.0, 42.1, 45.2, 68.1, 115.2, 118.5, 123.1, 128.5, 129.2, 129.4, 129.7, 130.6, 134.3, 151.9.
Representative procedure for the synthesis of sulfonamide analogs 3-8
A solution of N-(7-chloro-4-quinolyl)-N’-ethyl-1,4-diaminobutane (0.076 g, 0.27 mmol) and 2 (0.05 g, 0.14 mmol) in anhydrous DMF was heated at 90° C for 3 hours. After cooling to room temperature, DMF was removed in vacuo. Saturated NaHCO3 solution was added to the residue, which was then extracted with CH2Cl2, dried over anhydrous Na2SO4 and concentrated in vacuo. Flash chromatography using MeOH:CH2Cl2 (1:19 v/v) as the mobile phase gave 0.056 g (0.1 mmol, 75% yield) of 5 as a yellow oil. For the syntheses of 3, 4 and 7, the reactions were carried out in anhydrous THF and refluxed for 60 hours.
N-[2-{(N’-4-(7-chloro-4-quinolyl)aminobutyl-N”-ethyl}aminoethyl]-5-dimethylaminonaphthalene-1-sulfonamide, 5
1H-NMR (300 MHz, CDCl3) δ = 0.79 (t, J = 7.2 Hz, 3H), 1.44 (m, 2H), 1.64 (m, 2H), 2.22-2.34 (m, 4H), 2.43 (t, J = 6.0 Hz, 2H), 2.85 (s, 6H), 2.91 (t, J = 6.0 Hz, 2H), 3.24 (q, J = 6.0 Hz, 2H), 5.32 (bs, 1H), 6.37 (d, J = 5.4 Hz, 1H), 7.13 (dd, J = 0.9 Hz, J = 7.5 Hz, 1H), 7.32 (dd, J = 2.1 Hz, J = 8.7 Hz, 1H), 7.46-7.56 (m, 2H), 7.76 (d, J = 9.3 Hz, 1H), 7.94 (d, J = 2.4 Hz, 1H), 8.25 (dd, J = 1.2 Hz, J = 7.5 Hz, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.50-8.57 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ = 11.1, 24.6, 26.4, 40.4, 42.9, 45.3, 46.7, 51.5, 52.1, 98.8, 115.0, 117.1, 118.7, 121.5, 123.0, 125.1, 128.2, 129.5, 129.6, 129.7, 130.3, 134.3, 134.8, 148.7, 149.9, 151.6, 151.9.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-5-dimethylaminonaphthalene-1-sulfonamide, 3
Employing 0.16 g (0.44 mmol) of 2, 0.15 g (0.6 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-1,2-diaminoethane and 0.3 mL (1.7 mmol) of N,N-diisopropylethylamine in the procedure described above and purification by flash chromatography using MeOH:CH2Cl2 (1:19 v/v) as the mobile phase gave 0.18 g (0.33 mmol, 79% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 0.87 (t, J = 7.2 Hz, 3H), 2.42 (q, J = 7.2 Hz, 2H), 2.55 (t, J = 6.0 Hz, 2H), 2.68 (t, J = 6.0 Hz, 2H), 2.84 (s, 6H), 2.99 (t, J = 5.7 Hz, 2H), 3.19 (q, J = 5.7 Hz, 2H), 5.61 (bs, 2H), 6.25 (d, J = 5.4 Hz, 1H), 7.10 (d, J = 7.5 Hz, 1H), 7.28-7.50 (m, 3H), 7.73 (d, J = 9.0 Hz, 1H), 7.92 (d, J = 2.1 Hz, 1H), 8.20 (dd, J = 1.2 Hz, J = 7.2 Hz, 1H), 8.25 (d, J = 8.4 Hz, 1H), 8.45-8.56 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ = 10.9, 39.9, 40.8, 45.1, 46.6, 50.8, 52.0, 98.7, 114.9, 117.0, 118.4, 121.6, 122.9, 125.0, 127.7, 128.1, 129.1, 129.3, 129.6, 130.2, 134.5, 134.6, 148.4, 149.6, 151.5, 151.7.
N-[2-{(N’-3-(7-chloro-4-quinolyl)aminopropyl-N”-ethyl}aminoethyl]-5-dimethylaminonaphthalene-1-sulfonamide, 4
Employing 0.16 g (0.44 mmol) of 2 , 0.15 g (0.6 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-1,3-diaminopropane and 0.3 mL (1.7 mmol) of N,N-diisopropylethylamine in the procedure described above and purification by flash chromatography using MeOH:CH2Cl2 (1:19 v/v) as the mobile phase furnished 0.133 g (0.25 mmol, 57% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 0.88 (t, J = 7.2 Hz, 3H), 1.74 (m, 2H), 2.35-2.49 (m, 4H), 2.52 (t, J = 6.0 Hz, 2H), 2.84 (s, 6H), 2.97 (t, J = 6.0 Hz, 2H), 3.24 (q, J = 6.0 Hz, 2H), 5.48 (bs, 1H), 6.03 (bs, 1H), 6.30 (d, J = 5.4 Hz, 1H), 7.10 (d, J = 7.5 Hz, 1H), 7.28 (m, 1H), 7.40-7.52 (m, 2H), 7.61 (d, J = 8.7 Hz, 1H), 7.93 (t, J = 1.8 Hz, 1H), 8.21 (dd, J = 1.2 Hz, J = 7.5 Hz, 1H), 8.25 (d, J = 8.7 Hz, 1H), 8.47-8.55 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ = 11.0, 25.1, 40.6, 42.2, 45.2, 47.1, 51.5, 52.2, 98.5, 115.0, 177.1, 118.5, 121.6, 122.9, 124.9, 128.0, 128.2, 129.3, 129.4, 129.6, 130.3, 134.3, 134.5, 148.7, 150.0, 151.6, 151.8.
N-[2-{(N’-4-(7-chloro-4-quinolyl)aminopentyl-N”-ethyl}aminoethyl]-5-dimethylaminonaphthalene-1-sulfonamide, 6
Employing 0.063 g (0.17 mmol) of 2 and 0.1 g (0.34 mmol) of monodesethylchloroquine57 in the procedure described above and purification by flash chromatography using MeOH:CH2Cl2 (1:19 v/v) as the mobile phase afforded 0.058 g (0.10 mmol, 60% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 0.76 (t, J = 7.2 Hz, 3H), 1.31 (d, J = 6.3 Hz, 3H), 1.36-1.72 (m, 4H), 2.16-2.33 (m, 4H), 2.40 (m, 2H), 2.81-2.96 (m, 8H), 3.66 (m, 1H), 4.96 (d, J = 7.8 Hz, 1H), 5.51 (bs, 1H), 6.38 (d, J = 5.4 Hz, 1H), 7.13 (d, J = 7.5 Hz, 1H), 7.34 (m, 1H), 7.46-7.57 (m, 2H), 7.74 (d, J = 9.3 Hz, 1H), 7.95 (d, J = 1.8 Hz, 1H), 8.25 (m, 1H), 8.31 (d, J = 9.0 Hz, 1H), 8.48-8.58 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ = 11.2, 20.2, 23.6, 34.3, 40.3, 45.3, 46.6, 48.2, 51.5, 52.5, 99.0, 115.0, 117.1, 118.7, 121.3, 123.0, 125.0, 128.2, 128.4, 129.5, 129.6, 129.8, 130.5, 134.3, 134.7, 149.0, 149.1, 151.7, 151.9.
N-[2-{(N’-5-(7-chloro-4-quinolyl)aminopentyl-N”-ethyl}aminoethyl]-5-dimethylaminonaphthalene-1-sulfonamide, 7
Employing 0.064 g (0.17 mmol) of 2 and 0.1 g (0.34 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-1,5-diaminopentane in the procedure described above and purification by flash chromatography using MeOH:CH2Cl2 (1:19 v/v) as the mobile phase afforded 0.073 g (0.13 mmol, 75% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 0.76 (t, J = 7.2 Hz, 3H), 1.29-1.39 (m, 4H), 1.67 (m, 2H), 2.16-2.28 (m, 4H), 2.39 (t, J = 6.0 Hz, 2H), 2.82-2.93 (m, 8H), 3.28 (q, J = 6.0 Hz, 2H), 5.36 (t, J = 4.8 Hz, 1H), 6.37 (d, J = 5.7 Hz, 1H), 7.14 (d, J = 7.2 Hz, 1H), 7.26 (dd, J = 2.4 Hz, J = 9.0 Hz, 1H), 7.47-7.57 (m, 2H), 7.77 (d, J = 9.0 Hz, 1H), 7.93 (d, J = 2.4 Hz, 1H), 8.25 (dd, J = 1.5 Hz, J = 7.2 Hz, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.48-8.58 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ = 11.1, 24.9, 26.6, 28.4, 40.3, 43.1, 45.3, 46.6, 51.4, 52.3, 98.8, 115.0, 117.1, 118.7, 121.5, 123.0, 125.0, 128.1, 128.2, 129.5, 129.5, 129.7, 130.2, 134.2, 134.7, 148.8, 149.9, 151.6, 151.8.
N-[2-{(N’-6-(7-chloro-4-quinolyl)aminohexyl-N”-ethyl}aminoethyl]-5-dimethylaminonaphthalene-1-sulfonamide, 8
Employing 0.091 g (0.25 mmol) of 2 and 0.15 g (0.49 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-1,6-diaminohexane in the procedure described above and purification by flash chromatography using MeOH:CH2Cl2 (1:19 v/v) as the mobile phase provided 0.108 g (0.19 mmol, 76% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 0.74 (t, J = 7.2 Hz, 3H), 1.20-1.50 (m, 6H), 1.76 (m, 2H), 2.14-2.28 (m, 4H), 2.39 (t, J = 6.0 Hz, 2H), 2.81-2.94 (m, 8H), 3.31 (q, J = 7.2 Hz, 2H), 5.24 (bs, 1H), 6.40 (d, J = 5.4 Hz, 1H), 7.14 (d, J = 7.5 Hz, 1H), 7.28 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.48-7.58 (m, 2H), 7.74 (d, J = 9.0 Hz, 1H), 7.93 (d, J = 2.1 Hz, 1H), 8.25 (dd, J = 1.2 Hz, J = 7.2 Hz, 1H), 8.32 (d, J = 8.7 Hz, 1H), 8.49-8.58 (m, 2H); 13C-NMR (75 MHz, CDCl3) δ = 11.1, 26.6, 26.8, 26.9, 28.5, 40.3, 43.0, 45.2, 46.5, 51.4, 52.3, 98.7, 115.0, 117.1, 118.6, 121.5, 123.0, 124.9, 128.1, 129.5, 129.7, 130.2, 134.2, 134.6, 148.7, 150.0, 151.6, 151.8.
N-t-Boc N’-ethyl-N’-[2-(7-chloro-4-quinolyl)aminoethyl]-1,2-diaminoethane, 9
To a solution of N-(7-chloro-4-quinolyl)-N’-ethyl-1,2-diaminoethane (0.95 g, 3.8 mmol) and N-t-Boc-glycinal58 (1.1 g, 6.9 mmol) in anhydrous CH2Cl2, sodium triacetoxyborohydride (1.46 g, 6.9 mmol) was added at room temperature and stirred for 24 hours. The reaction mixture was quenched with water, basified with 10N NaOH and extracted with dichloromethane. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. Flash chromatography using MeOH:CH2Cl2 (1:24 v/v) as the mobile phase afforded 0.8 g (2.0 mmol, 54% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 1.05 (t, J = 7.2 Hz, 3H), 1.34 (s, 9H), 2.56-2.70 (m, 4H), 2.81 (t, J = 6.0 Hz, 2H), 3.15-3.36 (m, 4H), 5.25 (bs, 1H), 6.08 (s, 1H), 6.31 (d, J = 5.4 Hz, 1H), 7.30 (dd, J = 2.4 Hz, J = 9.0 Hz, 1H), 7.73 (d, J = 9.0 Hz, 1H), 7.92 (d, J = 2.4 Hz, 1H), 8.47 (d, J = 5.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.6, 28.1, 38.5, 39.8, 47.2, 51.2, 52.6, 78.9, 98.9, 117.1, 121.3, 125.0, 128.0, 134.5, 148.6, 149.7, 151.6, 155.9.
N-Ethyl-N-[2-(7-chloro-4-quinolyl)aminoethyl]-1,2-diaminoethane, 10
To a solution of 9 (0.25 g, 0.62 mmol) in anhydrous methanol, 2M HCl (3.1 mL, 6.2 mmol) was added at room temperature and stirred overnight. The solvents were removed in vacuo. The reaction mixture was basified with 10N NaOH, extracted with dichloromethane, dried over anhydrous Na2SO4 and concentrated in vacuo to 0.17 g (0.57 mmol, 88% yield) of a brown oil. 1H-NMR (300 MHz, CD3OD) δ = 1.43 (t, J = 7.2 Hz, 3H), 3.40-3.58 (m, 4H), 3.60-3.78 (m, 4H), 4.16 (m, 2H), 7.13 (d, J = 6.9 Hz, 1H), 7.73 (dd, J = 1.8 Hz, J = 9.0 Hz, 1H), 7.92 (d, J = 1.8 Hz, 1H), 8.53 (d, J = 6.9 Hz, 1H), 8.64 (d, J = 9.0 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.5, 39.6, 40.1, 47.2, 50.9, 55.5, 98.8, 117.2, 121.5, 124.7, 128.1, 134.3, 148.8, 149.8, 151.7.
Representative procedure for the synthesis of sulfonamide analogs 11-14
To a solution of N-(7-chloro-4-quinolyl)-N’-ethyl-N’-(2-aminoethyl)-1,2-diaminoethane 10 (0.055 g, 0.21 mmol) and Et3N (0.06 mL, 0.42 mmol) in anhydrous CH2Cl2, 6-phenoxypyridine-3-sulfonyl chloride (0.06 g, 0.21 mmol) was added and the mixture was stirred at room temperature for 1 hour. Saturated NaHCO3 solution was added to the reaction mixture, which was then extracted with CH2Cl2, dried over anhydrous Na2SO4, and concentrated in vacuo. Flash chromatography using MeOH:CH2Cl2 (1:49 v/v) and gradually changing the ratio of MeOH:CH2Cl2 to 1:19 (v/v) gave 0.065 g (0.12 mmol, 61% yield) of a yellow oil.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-2-phenoxy-5-pyridinesulfonamide, 11
1H-NMR (300 MHz, CDCl3) δ = 1.00 (t, J = 7.2 Hz, 3H), 2.60 (q, J = 7.2 Hz, 2H), 2.69 (t, J = 6.0 Hz, 2H), 2.79 (t, J = 6.0 Hz, 2H), 3.10 (t, J = 6.0 Hz, 2H), 3.18 (q, J = 6.0 Hz, 2H), 5.92 (bt, 1H), 6.09 (d, J = 5.4 Hz, 1H), 6.89 (d, J = 8.7 Hz, 1H), 7.09-7.16 (m, 1H), 7.21-7.30 (m, 2H), 7.37-7.46 (m, 2H), 7.64 (d, J = 9.0 Hz, 1H), 7.74 (d, J = 2.1 Hz, 1H), 8.02 (dd, J = 2.7 Hz, J = 8.7 Hz, 1H), 8.34 (d, J = 5.4 Hz, 1H), 8.63 (d, J = 2.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.1, 39.8, 40.9, 46.8, 50.9, 52.0, 98.6, 111.3, 116.8, 121.3, 121.7, 125.3, 125.5, 127.3, 129.7, 131.1, 134.8, 138.1, 147.3, 147.9, 149.6, 151.3, 152.8, 165.8.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-3-pyridinesulfonamide, 12
Employing 0.045 g (0.26 mmol) of 3-pyridinesulfonyl chloride and 0.075 g (0.26 mmol) of 10 in the procedure described above followed by flash chromatography using MeOH:CH2Cl2 (1:19 v/v) and gradually changing the ratio of MeOH:CH2Cl2 to 1:9 (v/v) afforded 0.06 g (0.13 mmol, 53% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 1.02 (t, J = 7.2 Hz, 3H), 2.61 (q, J = 7.2 Hz, 2H), 2.70 (t, J = 6.0 Hz, 2H), 2.80 (t, J = 6.0 Hz, 2H), 3.13 (t, J = 6.0 Hz, 2H), 3.19 (q, J = 6.0 Hz, 2H), 5.86 (bt, 1H), 6.11 (d, J = 5.4 Hz, 1H), 7.28 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.35 (m, 1H), 7.64 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 2.1 Hz, 1H), 8.05 (m, 1H), 8.36 (d, J = 5.4 Hz, 1H), 8.74 (dd, J = 1.8 Hz, J = 5.1 Hz, 1H), 9.06 (dd, J = 0.9 Hz, J = 2.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.1, 39.8, 40.9, 46.9, 50.9, 52.0, 98.8, 116.9, 121.6, 123.6, 125.3, 127.5, 134.4, 134.8, 136.8, 147.7, 148.1, 149.5, 151.5, 152.9.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-8-quinolinesulfonamide, 13
Employing 0.04 g (0.17 mmol) of quinoline-8-sulfonyl chloride and 0.05 g (0.17 mmol) of 10 in the procedure described above followed by flash chromatography using MeOH:CH2Cl2 (1:49 v/v) and gradually changing the ratio of MeOH:CH2Cl2 to 1:24 (v/v) generated 0.056 g (0.12 mmol, 67% yield) of yellow crystals. 1H-NMR (300 MHz, CDCl3) δ = 0.88 (t, J = 7.2 Hz, 3H), 2.42 (q, J = 7.2 Hz, 2H), 2.62 (t, J = 6.0 Hz, 2H), 2.75 (t, J = 6.0 Hz, 2H), 2.98 (q, J = 6.0 Hz, 2H), 3.22 (q, J = 6.0 Hz, 2H), 5.98 (t, J = 4.2 Hz, 1H), 6.28 (d, J = 5.4 Hz, 1H), 6.72 (t, J = 5.4 Hz, 1H), 7.29 (dd, J = 2.1 Hz, J = 8.7 Hz, 1H), 7.41 (dd, J = 5.2 Hz, J = 8.1 Hz, 1H), 7.59 (dd, J = 7.2 Hz, J = 8.1 Hz, 1H), 7.87 (d, J = 8.7 Hz, 1H), 7.90 (d, J = 2.1 Hz, 1H), 7.99 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H), 8.18 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H), 8.39 (dd, J = 1.5 Hz, J = 7.2 Hz, 1H), 8.48 (d, J = 5.4 Hz, 1H), 8.77 (dd, J = 1.5 Hz, J = 4.5 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 10.9, 39.9, 41.2, 46.3, 50.9, 52.1, 61.5, 98.8, 98.9117.1, 121.8, 122.0, 122.1, 125.4, 125.4, 125.5, 128.0, 128.5, 131.1, 133.3, 134.8, 135.2, 136.9, 142.9, 148.6, 149.7, 150.9, 151.5, 151.6.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonamide, 14
Employing 0.045 g (0.18 mmol) of 4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonyl chloride and 0.053 g (0.18 mmol) of 10 in the procedure described above followed by flash chromatography using MeOH:CH2Cl2 (1:16 v/v) as the mobile phase afforded 0.062 g (0.12 mmol, 68% yield) of a yellow oil. 1H-NMR (300 MHz, CDCl3) δ = 0.97 (t, J = 7.2 Hz, 3H), 2.54 (q, J = 7.2 Hz, 2H), 2.64 (t, J = 6.0 Hz, 2H), 2.72-2.81 (m, 5H), 3.04 (t, J = 6.0 Hz, 2H), 3.16-3.30 (m, 4H), 4.27 (t, J = 4.5 Hz, 2H), 5.80 (bs, 1H), 5.91 (bt, 1H), 6.22 (d, J = 5.4 Hz, 1H), 6.73 (d, J = 8.4 Hz, 1H), 7.06 (d, J = 2.1 Hz, 1H), 7.10 (dd, J = 2.1 Hz, J = 8.4 Hz, 1H), 7.33 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.76 (d, J = 9.0 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 8.44 (d, J = 5.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.2, 38.4, 40.1, 40.9, 46.9, 48.1, 51.0, 52.1, 64.8, 98.7, 110.3, 115.7, 117.0, 117.2, 121.8, 125.5, 127.6, 131.6, 135.0, 136.7, 147.5, 148.1, 149.8, 151.2.
Representative procedure for the synthesis of sulfonamide analogs 15-18
To a mixture of N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.15 g, 0.64 mmol) in 4.5 mL of anhydrous THF under nitrogen at room temperature was added triethylamine (0.084 g, 0.83 mmol) and dansyl chloride (0.21 g, 0.76 mmol). After stirring for 36 hours at room temperature, the mixture was quenched with water and extracted with dichloromethane. The combined organic layers were dried over anhydrous MgSO4, concentrated in vacuo, and purified by recrystallization from chloroform to give 15 as a white solid (0.06 g, 0.13 mmol, 20% yield).
N-(N’-3-(7-chloro-4-quinolyl)aminopropyl)-5-dimethylaminonaphthalene-1-sulfonamide, 15
1H-NMR (300 MHz, DMSO-d6) δ = 1.69 (tt, J = 6.6 Hz, J = 6.6 Hz, 2H), 2.80 (s, 6H), 2.94 (dt, J = 6.6 Hz, J = 6.1 Hz, 2H), 3.10 (dt, J = 6.6 Hz, J = 5.8 Hz, 2H), 6.16 (d, J = 7.3 Hz, 1H), 7.15 (t, J = 5.8 Hz, 1H), 7.23 (d, J = 7.9 Hz, 1H), 7.41 (dd, J = 2.2 Hz, J = 8.5 Hz, 1H), 7.54 (d, J = 7.9 Hz, 1H), 7.59 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 2.2 Hz, 1H), 7.99 (t, J = 6.1 Hz, 1H), 8.09 (dd, J = 1.0 Hz, J = 7.3 Hz, 1H), 8.15 (d, J = 7.3 Hz, 1H), 8.30 (m, 2 H), 8.41 (d, J = 7.3 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 27.8, 45.0, 98.4, 115.0, 117.3, 118.9, 123.43, 123.9, 127.3, 127.8, 128.3, 129.0, 129.3, 133.3, 135.8, 148.8, 149.80, 151.3, 151.6
N-(N’-3-(7-chloro-4-quinolyl)aminopropyl)-8-quinolinesulfonamide, 16
Employing 0.19 g (0.82 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 8-quinolinesulfonyl chloride (0.22 g, 0.98 mmol) in the procedure described above gave 0.14 g (0.33 mmol, 40% yield) of white crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.71 (tt, J = 6.5 Hz, J = 6.5 Hz, 2H), 2.95 (dt, J = 6.5 Hz, J = 5.8 Hz, 2H), 3.15 (dt, J = 6.5 Hz, J = 6.2 Hz, 2H), 6.23 (d, J = 5.5 Hz, 1H), 7.17 (t, J = 5.8 Hz, 1H), 7.35 (t, J = 6.2 Hz, 1H), 7.42 (dd, J = 2.2 Hz, J = 9.0 Hz, 1H), 7.65-7.74 (m, 2H), 7.76 (d, J = 2.2 Hz, 1H), 8.12 (d, J = 9.1 Hz, 1H), 8.24 (dd, J = 1.3 Hz, J = 8.3 Hz, 1H), 8.30-8.34 (m, 2H), 8.49 (dd, J = 1.8 Hz, J = 8.4 Hz, 1H), 9.03 (dd, J = 1.8 Hz, J = 4.2 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 27.6, 39.6, 40.7, 98.4, 117.2, 122.4, 124.0, 125.6, 127.1, 128.4, 130.6, 133.4, 133.5, 136.2, 136.9, 142.6, 148.6, 149.9, 151.2, 151.5.
N-(N’-3-(7-chloro-4-quinolyl)aminopropyl)-2-phenoxy-5-pyridinesulfonamide, 17
Employing 0.15 g (0.64 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 6-phenoxy-3-pyridinesulfonyl chloride (0.2 g, 0.76 mmol) in the procedure described above gave 0.038 g (0.081 mmol, 13% yield) of white crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.94 (tt, J = 6.2 Hz, J = 6.2 Hz, 2H), 3.16 (t, J = 6.2 Hz, 2H), 3.54 (dt, J = 6.2 Hz, 2H), 5.57 (bs, 1H), 6.32 (d, J = 5.7 Hz, 1H), 7.00 (d, J = 8.4 Hz, 1H), 7.13 (d, J = 8.4 Hz, 2H), 7.28 (d, J = 8.4 Hz, 1H), 7.36 (dd, J = 2.0 Hz, J = 8.9 Hz, 1H), 7.41-7.46 (m, 2H), 7.70 (d, J = 8.9 Hz, 1H), 7.90 (d, J = 1.9 Hz, 1H), 8.08 (dd, J = 2.7 Hz, J = 8.6 Hz, 1 H), 8.46 (d, J = 5.7 Hz, 1H), 8.64 (d, J = 2.7 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 28.5, 41.2, 99.4, 112.4, 118.1, 122.3, 124.8, 126.1, 128.1, 130.6, 132.4, 134.1, 139.4, 147.1, 149.6, 150.7, 152.5, 153.6, 165.8.
N-(N’-3-(7-chloro-4-quinolyl)aminopropyl)-4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonamide, 18
Employing 0.102 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 1,4-benzoxazinesulfonyl chloride (0.123 g, 0.49 mmol) in the procedure described above provided 0.02 g (0.04 mmol, 10% yield) of off-white crystals. 1H-NMR (400 MHz, CDCl3) δ = 1.79-1.86 (m, 2H), 2.76 (s, 3H), 3.02 (t, J = 4.5 Hz, 2H), 3.19 (t, J = 3.4 Hz, 2H), 3.44 (t, J = 4.5 Hz, 2H), 4.23 (t, J = 3.8 Hz, 2H), 5.79 (bs, 1H), 6.25 (d, J = 4.1 Hz, 1H), 6.72 (d, J = 6.0 Hz, 1H), 6.99 (d, J = 1.5 Hz, 1H), 7.06 (dd, J = 1.8 Hz, J = 6.6 Hz, 1H), 7.28 (dd, J = 1.8 Hz, J = 6.6 Hz, 1H), 7.72 (d, J = 6.6 Hz, 1H), 7.83 (d, J = 1.2 Hz, 1H), 8.37 (d, J = 4.1 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 26.8, 32.0, 37.5, 38.6, 39.5, 47.3, 63.9, 97.7, 109.3, 115.0, 116.3, 120.5, 124.5, 127.2, 134.2, 135.9, 146.8, 148.7, 150.4, 164.2.
Representative procedure for the synthesis of urea (19-22 and thiourea 23-26 analogs
A mixture of N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.15 g, 0.64 mmol) and the appropriate isothiocyanate or isocyanate (0.53 mmol) in anhydrous THF was stirred at room temperature until the reaction was complete. In all cases, the desired urea or thiourea product precipitated from solution. The precipitate was collected via vacuum filtration and dried in vacuo.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(4-methoxyphenyl)urea, 19
Employing 0.195 g (0.83 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 4-methoxyphenyl isocyanate (0.09 mL, 0.69 mmol) in the procedure described above gave 0.244 g (0.64 mmol, 89 % yield) of white crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.81-1.89 (m, 2H), 3.19-3.38 (m, 4H), 3.73 (s, 3H), 6.18 (t, J = 5.6 Hz, 1H), 6.52 (d, J = 5.6 Hz, 2H), 6.85 (dd, J = 2.1 Hz, J = 6.7 Hz, 2H), 7.31-7.37 (m, 3H), 7.49 (dd, J = 3.2 Hz, J = 10.0 Hz, 1H), 7.83 (d, J = 2.2 Hz, 1H), 8.30 (d, J = 2.7 Hz , 1H), 8.32 (s, 1H), 8.44 (d, J = 5.4 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 25.8, 29.3, 37.8, 55.8, 114.9, 118.2, 120.2, 124.8, 128.2, 134.1, 134.3, 149.8, 150.8, 152.7, 154.7, 156.4.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(2-methoxy-4-nitrophenyl)urea, 20
Employing 0.146 g (0.65 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 2-methoxy-4-nitrophenyl isocyanate (0.1 g, 0.52 mmol) in the procedure described above gave 0.186 g (0.43 mmol, 83 % yield) of yellow crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.85-1.90 (m, 2H), 3.25-3.31 (m, 3H), 3.61 (t, J = 6.4 Hz, 1H), 4.00 (s, 3H), 6.51 (d, J = 5.4 Hz, 1H), 7.32 (t, J = 5.3 Hz, 2H), 7.46 (dd, J = 2.2 Hz, J = 8.8 Hz, 1H), 7.78 (dd, J = 2.2 Hz, J = 10.7 Hz, 2H), 7.88 (dd, J = 2.4 Hz, J = 9.0 Hz, 1H), 8.29 (d, J = 9.3 Hz, 1H), 8.40 (d, J =3.4 Hz, 1H), 8.43 (s, 1H), 8.57 (s, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 28.9, 57.1, 79.9, 106.1, 116.4, 118.2, 118.4, 124.8, 128.2, 134.1, 137.4, 140.9, 147.2, 149.8, 150.7, 152.6, 155.2.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(4-dimethylaminophenyl)urea, 21
Employing 0.178 g (0.75 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 4-dimethylaminophenyl isocyanate (0.1 g, 0.62 mmol) in the procedure described above furnished 0.208 g (0.52 mmol, 84 % yield) of white crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.81-1.88 (m, 2H), 2.84 (s, 6H), 3.24 (q, J = 6.4 Hz, 2H), 3.31-3.39 (m, 2H), 6.11 (t, J = 5.7 Hz, 1H), 6.52 (d, J = 5.4 Hz, 1H), 6.69 (d, J = 9.0 Hz, 2H), 7.23 (d, J = 9.0 Hz, 2H), 7.37 (t, J = 5.0 Hz, 1H), 7.49 (dd, J = 2.2 Hz, J = 9.0 Hz, 1H), 7.83 (d, J = 2.2 Hz, 1H), 8.12 (s, 1H), 8.30 (d, J = 9.0 Hz, 1H), 8.44 (d, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 2.8, 29.4, 37.8, 67.7, 95.2, 99.3, 113.9, 116.2, 118.2, 120.6, 124.8, 128.2, 131.2, 134.1, 135.5, 138.2, 143.7, 146.8, 149.3, 150.7, 152.6, 156.6.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(2-methoxyphenyl)urea, 22
Employing 0.191 g (0.81 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 2-methoxyphenyl isocyanate (0.10 mL, 0.75 mmol) in the procedure described above gave 0.254 g (0.66 mmol, 82 % yield) of white crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.85-1.89 (m, 2H), 3.23-3.39 (m, 4H), 3.86 (s, 3H), 6.53 (d, J = 5.9 Hz, 1H), 6.84-7.02 (m, 4H), 7.36 (t, J = 5.1 Hz, 1H), 7.49 (dd, J = 2.2 Hz, J = 8.8 Hz, 1H), 7.83 (d, J = 2.2 Hz, 1H), 7.94 (s, 1H), 8.14 (dd, J = 2.0 Hz, J = 7.1 Hz, 1H), 8.32 (d, J = 9.0 Hz, 1H), 8.44 (d, J = 5.6 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 25.8, 29.2, 37.7, 67.7, 99.4, 118.2, 118.7, 121.7, 124.7, 128.2, 130.2, 134.1, 148.0, 149.8, 150.8, 152.6, 156.0.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(4-methoxyphenyl)thiourea, 23
Employing 0.162 g (0.69 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 4-methoxyphenyl isothiocyanate (0.08 mL, 0.58 mmol) in the procedure described above gave 0.116 g (0.29 mmol, 51 % yield) of white crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.95-1.99 (m, 2H), 3.35 (q, J = 6.3 Hz, 2H), 3.38 (bs, 2H), 3.77 (s, 3H), 6.52 (d, J = 5.4 Hz, 1H), 6.93 (dd, J = 2.2 Hz, J = 6.8 Hz, 2H), 7.25 (d, J = 9.0 Hz, 2H), 7.40 (t, J = 5.1 Hz, 1H), 7.50 (dd, J = 2.2 Hz, J = 9.0 Hz, 1H), 7.64 (bs, 1H), 7.83 (d, J = 2.2 Hz, 1H), 8.29 (d, J = 9.0 Hz, 1H), 8.44 (d, J = 5.4 Hz, 1H), 9.38 (bs, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 25.8, 28.2, 55.9, 67.7, 114.7, 118.2, 124.8, 126.8, 128.0, 132.3, 134.2, 149.6, 150.8, 152.4, 157.3, 181.4.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(2-methoxy-4-nitrophenyl)thiourea, 24
Employing 0.136 g (0.58 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 2-methoxy-4-nitrophenyl isothiocyanate (0.101 g, 0.48 mmol) in the procedure described above afforded 0.144 g (0.33 mmol, 76 % yield) of yellow crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.98-2.03 (m, 2H), 3.36-3.42 (m, 3H), 3.63-3.69 (m, 2H), 4.02 (s, 3H), 6.54 (d, J = 5.4 Hz, 1H), 7.37 (t, J = 5.3 Hz, 1H), 7.49 (dd, J = 2.4 Hz, J = 9.0 Hz, 1H), 7.83 (t, J = 2.4 Hz, 2H), 7.90 (dd, J = 2.7 Hz, J = 9.0 Hz, 3H), 8.31 (d, J = 9.3 Hz, 1H), 8.45 (d, J =5.4 Hz, 1H), 8.79 (d, J =9.0 Hz , 1H), 9.13 (bs, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 27.7, 42.5, 57.2, 79.9, 106.5, 116.8, 118.2, 124.8, 128.2, 134.1, 136.1, 142.9, 149.8, 150.7, 152.7, 180.5.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(4-dimethylaminophenyl)thiourea, 25
Employing 0.159 g (0.67 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 4-dimethylaminophenyl isothiocyanate (0.101 g, 0.57 mmol) in the procedure described above gave 0.157 g (0.38 mmol, 67 % yield) of white crystals. 1H-NMR (300 MHz, DMSO-d6) δ = 1.90-1.99 (m, 2H), 2.90 (s, 6H), 3.30-3.38 (m, 2H), 3.61-3.64 (m, 2H), 6.50 (d, J = 5.4 Hz, 1H), 6.71 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 8.8 Hz, 2H), 7.36 (t, J = 5.3 Hz, 1H), 7.49 (dd, J = 2.2 Hz, J = 9.0 Hz, 2H), 7.82 (d, J = 2.2 Hz, 1H), 8.27 (d, J = 9.0 Hz, 1H), 8.44 (d, J = 5.4, 1H), 9.28 (s, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 28.2, 79.9, 113.3, 118.2, 124.7, 126.7, 128.2, 134.0, 149.0, 149.8, 150.7, 152.6, 181.3.
N-(3-(7-chloro-4-quinolyl)aminopropyl)-N’-(4-dimethylaminonaphthyl)thiourea, 26
N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.123 g, 0.52 mmol) and 4-dimethylamino-1-naphthyl isothiocyanate (0.10 g, 0.44 mmol) were employed in the procedure described above. The solution was then cooled to -45 °C and 0.175 g (0.38 mmol, 96 % yield) of white crystals were obtained. 1H-NMR (300 MHz, DMSO-d6) δ = 1.92 (bs, 2H), 2.87 (s, 6H), 3.28 (bs, 2H), 3.59-3.66 (m, 2H), 6.43 (s, 1H), 7.12 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.1 Hz, 2H), 7.48 (dd, J = 2.2 Hz, J = 9.0 Hz, 1H), 7.54-7.57 (m, 2H), 7.81-7.87 (m, 2H), 8.20-8.27 (m, 2H), 8.40 (d, J = 5.6 Hz, 1H), 9.57 (bs, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 28.1, 45.6, 79.9, 99.3, 118.2, 124.7, 126.1, 128.2, 129.5, 130.4, 132.1, 134.1, 149.8, 150.6, 151.6, 152.6, 167.4, 182.5.
N-(7-Chloro-4-quinolyl)-N’-(3-diethylaminopropanoyl)-1,2-diaminoethane, 27
A mixture of N-(7-chloro-4-quinolyl)-1,2-diaminoethane (0.1 g, 0.45 mmol), N,N-diethylamino-3-propionic acid (0.11 g, 0.6 mmol), EDC ( 0.11 g, 0.6 mmol) and Et3N (0.19 mL, 1.35 mmol) in 4 mL of anhydrous DMF and CHCl3 (1:1 v/v) was stirred at room temperature for 2 days. Saturated NaHCO3 solution was added to the cooled reaction mixture, which was then extracted with CH2Cl2, dried over anhydrous MgSO4, and concentrated in vacuo. Flash chromatography using EtOH:Et3N (1:0.05 v/v) as the mobile phase afforded 0.10 g (0.44 mmol, 63% yield) of yellow crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.05 (t, J = 7.1 Hz, 6H), 2.48 (t, J = 6.1 Hz, 2H), 2.58 (q, J = 7.1 Hz, 4H), 2.69 (t, J = 6.1 Hz, 2H), 3.30-3.45 (m, 2H), 3.64-3.78 (m, 2H), 6.28 (d, J = 5.4 Hz,1H), 7.11 (bs, 1H), 7.40 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.87 (d, J = 9.0 Hz, 1H), 7.94 (d, J = 2.1 Hz, 1H), 8.50 (d, J = 5.4 Hz, 1H), 9.51 (bs, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.6, 32.4, 38.4, 46.3, 46.5, 48.9, 98.2, 117.5, 122.7, 125.7, 128.2, 135.2, 148.9, 150.7, 151.8, 176.2.
N-(7-Chloro-4-quinolyl)-N’-(3-diethylaminopropanoyl)-1,3-diaminopropane, 28
A mixture of N-(7-chloro-4-quinolyl)-1,3-diaminopropane (1.0 g, 4.24 mmol), N,N-diethylamino-3-propionic acid (0.78 g, 4.3 mmol), EDC (0.98 g, 5.1 mmol) and triethylamine (1.8 mL, 12.9 mmol) in 30 mL of anhydrous DMF and chloroform (1:1 v/v) was stirred at room temperature for 2.5 days. The reaction mixture was concentrated in vacuo, then dissolved in dichloromethane and extracted with aqueous NaOH. The combined organic layers were dried over anhydrous MgSO4 and concentrated in vacuo. The crude product was purified by flash chromatography (ethanol:hexanes:triethylamine 1:1:0.05 v/v) to give 0.83 g of (2.3 mmol, 54% yield) pale yellow crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.02 (t, J = 7.1 Hz, 6H), 1.74-1.83 (m, 2H), 2.41 (t, J = 5.7 Hz, 2H) , 2.53 (q, J = 7.1 Hz, 4H), 2.67 (t, J = 5.9 Hz, 2H), 3.32-3.43 (m, 4H), 6.37 (d, J = 5.6 Hz, 1H), 6.76 (t, J = 5.7 Hz, 1H), 7.36 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.90 (d, J = 2.1 Hz, 1H), 8.02 (d, J = 9.0 Hz, 1H), 8.45 (d, J = 5.6 Hz, 1H), 9.04 (t, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.8, 28.6, 32.7, 35.7, 39.2, 46.5, 49.2, 98.6, 117.9, 122.5, 125.7, 128.5, 135.4, 149.4, 150.5, 151.9, 174.8.
N-(7-Chloro-4-quinolyl)-N’-(3-diethylaminopropanoyl)-1,4-diaminobutane, 29
A mixture of N-(7-chloro-4-quinolyl)-1,4-diaminobutane (2.0 g, 8.0 mmol), N,N-diethylamino-3-propionic acid (1.45 g, 8.0 mmol), EDC (1.84 g, 9.6 mmol), and triethylamine (3.35 mL, 24.0 mmol) in 80 mL of anhydrous DMF and chloroform (1:1 v/v) was stirred at room temperature for 2.5 days. The reaction mixture was concentrated in vacuo and partitioned between dichloromethane and 1N NaOH solution. The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by flash chromatography (methanol:ammonium hydroxide 1.0:0.005 v/v) to give 1.8 g (4.8 mmol, 60% yield) of colorless crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.02 (t, J = 7.2 Hz, 6H), 1.60-1.88 (m, 4H), 2.36 (t, J = 6.0 Hz, 2H), 2.54 (q, J = 7.2 Hz, 4H), 2.65 (t, J = 6.0 Hz, 2H), 3.28-3.42 (m, 4H), 5.71 (bt, 1H), 6.38 (d, J = 5.7 Hz, 1H), 7.35 (dd, J = 2.4 Hz, J = 9.0 Hz, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.93 (d, J = 2.4 Hz, 1H), 8.51 (d, J = 5.7 Hz, 1H), 8.85 (bt, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.3, 25.2, 27.8, 32.3, 38.1, 42.9, 45.8, 48.6, 98.6, 117.3, 121.9, 124.7, 128.0, 134.4, 148.9, 150.0, 151.6, 173.1.
N-(7-Chloro-4-quinolyl)-N’-(3-diethylaminopropanoyl)-1,5-diaminopentane, 30
A mixture of N-(7-chloro-4-quinolyl)-1,5-diaminopentane (0.25 g, 0.95 mmol), N,N-diethylamino-3-propionic acid (0.17 g, 0.93 mmol), EDC (0.22 g, 1.14 mmol), and triethylamine (0.4 mL, 2.9 mmol) in 12 mL of anhydrous DMF and chloroform (1:1 v/v) was stirred at room temperature for 2.5 days. The reaction mixture was concentrated in vacuo, then dissolved in dichloromethane and extracted with aqueous NaOH. The combined organic layers were dried over anhydrous MgSO4 and concentrated in vacuo. The crude product was purified by flash chromatography (methanol:ammonium hydroxide 1.0:0.05 v/v) to afford 0.045 g (0.11 mmol, 12% yield) of colorless crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.01 (t, J = 7.2 Hz, 6H), 1.49-1.59 (m, 4H), 1.82-1.87 (m, 2H) , 2.53 (t, J = 6.0 Hz, 2H), 2.53 (q, J = 7.2 Hz, 4H), 2.64 (t, J = 6.0 Hz, 2H), 3.26-3.33 (m, 4H), 5.46 (bs, 1H), 6.37 (d, J = 5.4 Hz, 1H), 7.35 (dd, J = 2.2 Hz, 8.8 Hz, 1H), 7.94 (d, J = 2.2 Hz, 1H), 7.96 (d, J = 8.8 Hz, 1H), 8.51 (d, J = 5.4 Hz, 1H), 8.80 (bs, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.8, 24.3, 28.0, 30.0, 32.8, 37.9, 43.4, 46.3, 49.2, 100.6, 117.6, 122.0, 128.9, 134.9, 149.5, 150.3, 152.3, 173.7.
N-(7-Chloro-4-quinolyl)-N’-(3-diethylaminopropanoyl)-1,6-diaminohexane, 31
A mixture of N-(7-chloro-4-quinolyl)-1,6-diaminohexane (0.1 g, 0.36 mmol), N,N- diethylamino-3-propionic acid (0.08 g, 0.43 mmol), EDC ( 0.08 g, 0.43 mmol) and Et3N (0.19 mL, 1.35 mmol) was stirred at room temperature in 4 mL of DMF:CHCl3 (1:1 v/v) for 2 days. Saturated NaHCO3 was added to the cooled reaction mixture, which was then extracted with CH2Cl2 and dried over anhydrous MgSO4, and concentrated in vacuo. Purification by flash chromatography using EtOH:Et3N (1:0.05 v/v) as the mobile phase gave yellow crystals (0.12 g, 0.27 mmol, 76% yield). 1H-NMR (300 MHz, CDCl3) δ =1.06 (t, J = 7.1 Hz, 6H), 1.25-1.62 (m, 6H), 1.63-1.82 (m, 2H), 2.40 (t, J = 6.1 Hz, 2H), 2.58 (q, J = 7.1 Hz, 4H), 2.69 (t, J = 6.1 Hz, 2H), 3.20-3.41 (m, 4H), 5.37 (bs, 1H), 6.41 (d, J = 5.4 Hz, 1H), 7.38 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 2.1 Hz, 1H), 8.53 (d, J = 5.4 Hz, 1H), 8.67 (bs, 1H); 13C-NMR (75 MHz, CDCl3) δ = 11.7, 26.7, 28.7, 29.8, 32.7, 38.7, 43.1, 46.3, 49.2, 99.1, 117.5, 121.9, 125.3, 128.6, 135.0, 149.2, 150.3, 152.0, 173.2.
Representative procedure for the synthesis of amide analogs 32-36
To a solution of N-(1-naphthyl)anthranilic acid (0.15 g, 0.57 mmol) and 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) (0.1 g, 0.57 mmol) in anhydrous CHCl3, 0.1 mL (0.63 mmol) of N-methylmorpholine (NMM) was added dropwise at 0 °C and stirred at room temperature for 2 hours. N-(7-Chloro-4-quinolyl)-1,3-diaminopropane (0.41 g, 1.7 mmol) in anhydrous DMF was then added. The reaction mixture was stirred for another 2 hours and concentrated under reduced pressure. The residue was dissolved in CH2Cl2, extracted with water, dried over anhydrous Na2SO4 and concentrated in vacuo. Flash chromatography (MeOH:EtOAc 1:32 v/v) allowed the isolation of 0.2 g of 32 (0.41 mmol, 73% yield) as light brown crystals.
N-(7-Chloro-4-quinolyl)-N’-(2-naphthylaminobenzoyl)-1,3-diaminopropane, 32
1H-NMR (300 MHz, CDCl3) δ = 1.91 (m, 2H), 3.37 (q, J = 5.7 Hz, 2H), 3.58 (q, J = 6.3 Hz, 2H), 6.32 (d, J = 5.7 Hz, 1H), 6.46 (t, J = 5.7 Hz, 1H), 6.71 (m, 1H), 6.97 (bs, 1H), 7.16-7.28 (m, 3H), 7.38-7.56 (m, 5H), 7.62 (d, J = 8.1 Hz, 1H), 7.84-7.95 (m, 3H), 8.17 (m, 1H), 8.42 (d, J = 5.7 Hz, 1H), 9.90 (s, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 27.6, 37.1, 40.2, 98.5, 98.7, 114.8, 115.7, 117.4, 117.8, 118.1, 121.4, 122.6, 124.0, 126.0, 126.2, 127.2, 127.3, 128.4, 128.7, 131.9, 133.4, 134.3, 137.1, 145.4, 148.8, 150.1, 151.6, 169.3.
N-(7-Chloro-4-quinolyl)-N’-(2-benzylamino-4-fluorobenzoyl)-1,3-diaminopropane, 33
Employing 0.15 g (0.6 mmol) of 4-fluoro-N-benzylanthranilic acid and N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.43 g, 1.83 mmol) in the procedure described above and purification by flash chromatography (MeOH:EtOAc 1:49 v/v) gave 0.16 g (0.35 mmol, 59% yield) of colorless crystals. 1H-NMR (300 MHz, CD3OD) δ = 2.00 (m, 2H), 3.40-3.54 (m, 4H), 4.31 (s, 2H), 6.23-6.38 (m, 2H), 6.52 (d, J = 5.7 Hz, 1H), 7.18-7.38 (m, 6H), 7.50 (dd, J = 2.1 Hz, 9.0 Hz, 1H), 7.75 (d, J = 2.1 Hz, 1H), 8.05 (d, J = 9.0 Hz, 1H), 8.31 (d, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ = 27.7, 36.8, 36.9, 45.9, 46.0, 97.5, 97.9, 98.6, 100.8, 101.1, 111.8, 117.4, 117.5, 124.0, 126.9, 127.1, 127.4, 128.5, 130.6, 130.8, 133.3, 138.9, 149.0, 149.9, 150.0, 151.1, 151.2, 151.3, 151.4, 151.8, 163.3, 166.5, 168.4, 168.4, 168.5.
N-(7-Chloro-4-quinolyl)-N’-(2-phenylethylaminobenzoyl)-1,3-diaminopropane, 34
Employing 0.15 g (0.6 mmol) of N-phenethylanthranilic acid and N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.44 g, 1.86 mmol) in the procedure described above and purification by flash chromatography (MeOH:EtOAc 1:24 v/v) gave 0.19 g (0.42 mmol, 68% yield) of colorless crystals. 1H-NMR (300 MHz, CD3OD) δ = 1.98 (m, 2H), 2.90 (t, J = 7.2 Hz, 2H), 3.36-3.52 (m, 6H), 6.50 (d, J = 5.7 Hz, 1H), 6.59 (m, 1H), 6.74 (d, J = 8.1 Hz, 1H), 7.13 (m, 1H), 7.20-7.48 (m, 7H), 7.78 (d, J = 2.4 Hz, 1H), 8.06 (d, J = 9.0 Hz, 1H), 8.33 (d, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ =22.7, 34.8, 36.8, 43.8, 43.9, 79.1, 98.6, 111.0, 114.1, 115.0, 115.1, 115.2, 117.4, 117.5, 124.0, 126.0, 127.4, 128.2, 128.8, 132.3, 133.4, 139.4, 148.7, 148.8, 148.9, 149.9, 150.0, 151.7, 169.1, 169.1, 169.2, 169.2.
N-(7-Chloro-4-quinolyl)-N’-(2-cyclohexylthiobenzoyl)-1,3-diaminopropane, 35
Employing 0.12 g (0.51 mmol) of 2-(cyclohexylthio)benzoic acid and N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.1 g, 0.43 mmol) in the procedure described above (this reaction was conducted at 70 °C) and purification by flash chromatography (MeOH:CH2Cl2 1:49 v/v and gradually changing the ratio of MeOH:CH2Cl2 to 1:11.5 v/v) gave 0.07 g (0.15 mmol, 35% yield) of light yellow crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.12-1.44 (m, 5H), 1.59 (s, 1H), 1.72 (m, 2H), 1.85-2.04 (m, 4H), 3.11 (m, 1H), 3.50-3.70 (m, 4H), 6.41 (d, J = 5.4 Hz, 1H), 6.77 (bt, 1H), 7.29-7.53 (m, 5H), 7.75 (dd, J = 7.5 Hz, J = 2.1 Hz, 1H), 7.90 (d, J = 2.1 Hz, 1H), 8.02 (d, J = 9.0 Hz, 1H), 8.46 (d, J = 5.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 25.2, 25.8, 28.1, 33.1, 36.5, 39.0, 48.0, 98.2, 98.3, 117.5, 122.2, 125.3, 127.4, 127.8, 129.2, 130.5, 132.0, 134.0, 135.0, 138.0, 148.7, 150.2, 151.2, 151.3, 169.6.
N-(7-Chloro-4-quinolyl)-N’-(2-phenylthiobenzoyl)-1,3-diaminopropane, 36
Employing 0.12 g (0.51 mmol) of 2-(phenylthio)benzoic acid and N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.1 g, 0.43 mmol) in the procedure described above (this reaction was conducted at 70 °C) and purification by flash chromatography (MeOH:CH2Cl2 1:24 v/v) gave 0.05 g (0.1 mmol, 25% yield) of light yellow crystals. 1H-NMR (300 MHz, CDCl3) δ = 1.84 (m, 2H), 3.42 (q, J = 6.0 Hz, 2H), 3.54 (q, J = 6.0 Hz, 1H), 6.36 (d, J = 5.4 Hz, 1H), 6.60 (t, J = 6.0 Hz, 1H), 6.89 (t, J = 6.0 Hz, 1H), 7.22-7.39 (m, 9H), 7.66 (m, 1H), 7.89 (d, J = 1.8 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.44 (d, J = 5.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 28.0, 36.6, 38.8, 98.2, 98.3, 117.5, 122.1, 125.2, 125.3, 127.3, 127.7, 128.0, 128.1, 128.8, 129.5, 131.0, 131.2, 132.3, 133.7, 134.3, 134.9, 136.5, 148.9, 150.0, 151.4, 151.5, 169.3.
Representative procedure for the synthesis of amide analogs (37-44)
N-(7-Chloro-4-quinolyl)-1,3-diaminopropane (0.1 g, 0.43 mmol), Boc-Trp-OH (0.16 g, 0.52 mmol) and 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) (0.09 g, 0.52 mmol) were dissolved in 3 mL of acetonitrile and 1 mL of DMF. N-Methylmorpholine (NMM) (0.165 g, 0.65 mmol) was added and the reaction was stirred at 40 °C for 24 hours. The solvents were removed under reduced pressure and dissolved in 25 mL of CH2Cl2 and washed twice with 1 mL of water and brine, respectively. The combined organic layers were dried over anhydrous MgSO4 and concentrated in vacuo. Purification by flash chromatography using EtOAc:EtOH:Et3N (4:1:0.02 v/v) as the mobile phase gave 0.134 g of 37 as a colorless oil (0.26 mmol, 60% yield) from Boc-D-Trp-OH. The same procedure gave 0.09 g of 38 as a colorless oil (0.17 mmol, 40% yield) from Boc-Trp-OH.
N-(7-Chloro-4-quinolyl)-N’-1,3-diaminopropan-N”-t-Boc-tryptophan amide, 37 and 38
1H-NMR (300 MHz, CDCl3) δ = 1.40 (s, 9H), 2.74-2.92 (m, 4H), 1.65-1.84 (m, 2H), 3.18-3.36 (m, 6H), 4.34 (t, J = 6.0 Hz, 1H), 6.36 (d, J = 5.6 Hz, 1H), 6.89-7.11 (m, 3H), 7.12 (s, 1H), 7.31 (d, J = 7.5 Hz, 1H), 7.40 (dd, J = 2.0 Hz, J = 7.2 Hz,1H), 7.77 (d, J = 2.0 Hz, 1H), 8.06 (d, J = 9.0 Hz, 1H), 8.31 (d, J = 6.0 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 27.5, 28.2, 36.6, 39.7, 56.2, 78.3, 79.5, 98.5, 109.8, 111.2, 117.6, 118.3, 118.7, 121.3, 123.1, 123.4, 124.8, 126.4, 127.7, 135.1, 136.9, 148.4, 151.3, 156.4, 174.0.
N-(7-Chloro-4-quinolyl)-N’-1,3-diaminopropan-N”-Z-lysine amide, 39
Employing 0.1 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and Z-Lys(Boc)-OH (0.198 g, 0.52 mmol) in the procedure described above and purification by flash chromatography using EtOAc:EtOH:Et3N (1:1:0.02 v/v) as the mobile phase gave a colorless oil (0.077 g, 0.13 mmol, 30% yield). 1H-NMR (300 MHz, CDCl3) δ = 1.42 (s, 9H), 1.61-1.98 (m, 6H), 2.95 (m, 1H), 3.01-3.18 (m, 2H), 3.22-3.45 (m, 4H), 4.11-4.25 (m, 1H), 4.83 (t, J = 6.0 Hz, 1H), 5.10 (s, 2H), 6.12 (d, J = 6.3 Hz, 1H), 6.48 (d, J = 5.6 Hz,1H), 7.25-7.40 (m, 5H), 7.92 (d, J = 2.2 Hz, 1H), 7.93 (d, J = 9.0 Hz, 1H), 8.42 (d, J = 5.6 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 22.8, 28.2, 28.7, 29.9, 31.9, 36.5, 39.3, 39.8, 55.6, 67.5, 79.6, 98.7, 117.8, 122.4, 125.5, 128.3, 128.5, 128.8, 135.1, 136.4, 149.4, 150.3, 152.2, 156.7, 156.8, 162.9, 173.7.
N-(7-Chloro-4-quinolyl)-N’-1,3-diaminopropan-N”-t-Boc-proline amide, 40
Employing 0.1 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and Boc-Pro-OH (0.112 g, 0.52 mmol) in the procedure described above and purification by flash chromatography using EtOH:Et3N (1:0.02 v/v) as the mobile phase gave a colorless oil (0.092 g, 0.24 mmol, 55% yield). 1H-NMR (300 MHz, CDCl3) δ = 1.45 (s, 9H), 1.82-2.10 (m, 6H), 3.36-3.62 (m, 6H), 4.18-4.22 (m, 1H), 6.62 (d, J = 9.0 Hz, 1H), 7.44 (dd, J = 2.2 Hz, J = 6.8 Hz, 1H), 7.81 (d, J = 2.2 Hz, 1H), 8.17 (d, J = 9.0 Hz, 1H), 8.38 (d, J = 6.8 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 24.6, 28.1, 28.4, 36.0, 38.9, 47.2, 60.3, 80.6, 98.2, 117.4, 122.2, 125.5, 127.6, 135.3, 148.3, 150.4, 150.9.
N-(7-Chloro-4-quinolyl)-N’-(3-pyridoyl)-1,3-diaminopropane, 41
Employing 0.1 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 3-nicotinic acid (0.064 g, 0.52 mmol) in the procedure described above and purification by flash chromatography using EtOH:Et3N (1:0.02 v/v) as mobile phase afforded a colorless oil (0.032 g, 0.084 mmol, 20% yield). 1H-NMR (300 MHz, CDCl3) δ = 2.01-2.72 (m, 2H), 3.51 (t, J = 6.9 Hz, 2H), 3.75 (t, J = 6.9 Hz, 2H), 6.66 (d, J = 8.4 Hz, 1H), 7.45-7.60 (m, 2H), 7.82 (dd, J = 2.1 Hz, J = 5.8 Hz, 1H), 8.24-8.31 (m, 2H), 8.39 (d, J = 2.1 Hz, 1H), 8.71 (dd, J = 2.1 Hz, J = 5.8 Hz, 1H), 9.10 (d, J = 8.4 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 28.2, 38.7, 39.5, 98.2, 116.3, 119.4, 123.6, 124.7, 127.3, 129.5, 133.9, 134.1, 134.7, 147.4, 147.8, 151.0, 151.7, 166.4.
N-(7-Chloro-4-quinolyl)-N’-[3-(6-hydroxypyridoyl]-1,3-diaminopropane, 42
Employing 0.1 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 6-hydroxynicotinic acid (0.075 g, 0.52 mmol) in the procedure described above and purification by flash chromatography using EtOH:Et3N (1:0.02 v/v) as mobile phase gave a colorless oil (0.038 g, 0.11 mmol, 25% yield). 1H-NMR (300 MHz, CDCl3) δ = 1.82-1.98 (m, 2H), 3.62 (t, J = 6.7 Hz, 2H), 3.79 (t, J = 6.7 Hz, 2H), 6.42 (d, J = 7.5 Hz, 1H), 7.15 (d, J = 6.8 Hz, 1H), 7.24-7.38 (m, 2H), 8.21 (d, J = 7.5 Hz, 1H), 8.32 (d, J = 6.8 Hz, 1H), 8.42 (dd, J = 2.0 Hz, J = 5.4 Hz, 1H), 8.91 (d, J = 2.0 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 28.4, 38.3, 39.6, 98.2, 110.9, 114.7, 119.4, 116.5, 124.6, 129.5, 134.0, 145.2, 149.8,151.8, 154.1, 154.7, 166.7.
N-(7-Chloro-4-quinolyl)-N’-(3-dimethylaminobenzoyl)-1,3-diaminopropane, 43
Employing 0.1 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 3-dimethylaminobenzoic acid (0.086 g, 0.52 mmol) in the procedure described above and purification by flash chromatography using CH2Cl2:EtOH:Et3N (1:1:0.02 v/v) as the mobile phase gave a colorless oil (0.042 g, 0.11 mmol, 25% yield). 1H-NMR (300 MHz, CDCl3) δ = 1.95-2.18 (m, 2H), 3.47 (t, J = 6.8 Hz, 2H), 3.55 (t, J = 6.8 Hz, 2H), 3.96 (s, 6H), 6.55 (d, J = 5.7 Hz, 1H), 7.36-7.42 (m, 2H), 7.53 (dd, J = 6.3 Hz, J = 7.5 Hz, 1H), 7.70 (dd, J = 2.1 Hz, J = 6.3 Hz, 1H), 7.75 (d, J = 2.1 Hz, 2H), 8.12 (d, J = 9 Hz, 1H), 8.32 (d, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 27.8, 39.4, 39.6, 40.7, 40.9, 98.7, 108.5, 116.3, 116.9, 120.6, 129.1, 134.4, 134.8, 148.5, 150.9, 151.7, 166.8.
N-(7-Chloro-4-quinolyl)-N’-[3-(2-benzimidazol)propanoyl]-1,3-diaminopropane, 44
Employing 0.1 g (0.43 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 2-benzimidazolepropionic acid (0.1 g, 0.52 mmol) in the procedure described above and purification by flash chromatography using EtOH:Et3N (1:0.01 v/v) as mobile phase gave a colorless oil (0.061 g, 0.15 mmol, 35% yield) . 1H-NMR (300 MHz, MeOD) δ = 1.78-1.95 (m, 2H), 2.27 (t, J = 7.2 Hz, 2H), 3.21 (t, J = 7.5 Hz, 2H), 3.25 (t, J = 7.5 Hz, 2H), 3.27 (t, J = 7.2 Hz, 2H), 6.38 (d, J = 5.7 Hz, 1H), 7.10-7.18 (m, 2H), 7.37 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.42-7.51 (m, 2H), 8.05 (d, J = 9.0 Hz, 1H), 7.77 (d, J = 2.1 Hz, 1H), 8.28 (d, J = 5.7 Hz, 1H); 13C-NMR (75 MHz, CDCl3) δ = 24.5, 27.8, 33.5, 36.6, 39.9, 98.4, 117.4, 122.1, 123.3, 125.1, 125.4, 135.4, 135.7, 147.2, 150.1, 152.0, 154.3, 173.3.
N-(7-Chloro-4-quinolyl)-N’-(2,5-diaminobenzoyl)-1,3-diaminopropane, 45
A mixture of 5-aminoisatoic anhydride (0.1 g, 0.56 mmol) and N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.15 g, 0.67 mmol) in ethanol was refluxed for 24 hours. After cooling to room temperature, the filtrate was concentrated under reduced pressure. The residue was purified using flash chromatography (MeOH:CH2Cl2 3:7 v/v) to afford 0.13 g (0.34 mmol, 64% yield) of brown crystals. 1H-NMR (300 MHz, CD3OD) δ = 1.95 (m, 2H), 3.36 (t, J = 6.9 Hz, 1H), 3.43 (t, J = 6.9 Hz, 1H), 6.42 (d, J = 6.0 Hz, 1H), 6.65 (d, J = 8.7 Hz, 1H), 6.74 (dd, J = 2.4 Hz, J = 8.7 Hz, 1H), 6.85 (d, J = 2.4 Hz, 1H), 7.29 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.70 (d, J = 2.1 Hz, 1H), 8.01 (d, J = 9.0 Hz, 1H), 8.26 (d, J = 6.0 Hz, 1H); 13C-NMR (75 MHz, CD3OD) δ = 29.2, 38.1, 41.3, 99.5, 115.9, 118.6, 119.8, 120.0, 122.4, 124.2, 125.9, 127.2, 136.3, 139.0, 141.8, 149.1, 152.0, 152.5, 172.1.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-2-benzylamino-4-fluorobenzamide, 46
Employing 0.034 g (0.14 mmol) of 4-fluoro-N-benzylanthranilic acid and N-(7-chloro-4-quinolyl)-N’-ethyl-N’-(2-aminoethyl)-1,2-diaminoethane 10 (0.04 g, 0.14 mmol) in the procedure described for the syntheses of 32-36 followed by flash chromatography using MeOH:CH2Cl2 (1:49 to 1:15 v/v) as the mobile phase gave 0.047 g (0.09 mmol, 66% yield) of a light yellow oil. 1H-NMR (300 MHz, CD3OD) δ = 1.10 (t, J = 6.9 Hz, 3H), 2.64-2.78 (m, 4H), 2.85 (t, J = 6.3 Hz, 2H), 3.34-3.47 (m, 4H), 3.55 (t, J = 6.9 Hz, 2H), 6.40 (d, J = 5.7 Hz, 1H), 7.24 (dd, J = 1.8 Hz, J = 9.0 Hz, 1H), 7.66 (d, J = 1.8 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.08 (s, 1H), 8.24 (d, J = 5.7 Hz, 1H), 8.38 (s, 2H); 19F-NMR (282 MHz, CDCl3) δ = -62.9; 13C-NMR (75 MHz, CD3OD) δ = 12.2, 38.5, 41.5, 47.8, 52.4, 53.5, 98.8 (m), 99.8, 99.9, 102.5 (m), 113.1, 118.5, 123.9, 126.0, 127.2, 127.3, 128.2, 129.6, 131.2 (dd, JC-F = 6.8 Hz, JC-F = 22.6 Hz), 136.4, 136.9, 149.0, 152.0, 152.5, 152.6, 152.7, 165.4, 168.7, 171.3.
Representative procedure for the synthesis of amide analogs 47-50
To a solution of N-(7-chloro-4-quinolyl)-1,3-diaminopropane (0.11 g, 0.47 mmol) and Et3N (0.13 mL, 0.94 mmol) in anhydrous DMF and CHCl3 (1:1 v/v), 3,5-bis(trifluoromethyl)benzoyl chloride (0.092 mL, 0.51 mmol) was added at 0 °C. The reaction mixture was stirred for 3 hours at room temperature and concentrated under reduced pressure. Saturated NaHCO3 solution was added to the residue, which was then extracted with CH2Cl2, dried over anhydrous Na2SO4, and concentrated in vacuo. Flash chromatography using MeOH:CH2Cl2 (1:19 to 1:9 v/v) gave 0.224 g of the N-acyl quinolinium salt of 47. The crystalline residue was hydrolyzed in 1N NaOH solution, extracted with CH2Cl2, dried over anhydrous Na2SO4, and concentrated in vacuo to yield 0.13 g (0.27 mmol, 59% yield) of off-white crystals.
N-(7-Chloro-4-quinolyl)-N’-{bis(trifluoromethyl)benzoyl}-1,3-diaminopropane, 47
1H-NMR (300 MHz, CD3OD) δ = 2.03 (m, 2H), 3.37 (t, J = 6.9 Hz, 2H), 3.55 (t, J = 6.9 Hz, 2H), 6.40 (d, J = 5.7 Hz, 1H), 7.24 (dd, J = 1.8 Hz, J = 9.0 Hz, 1H), 7.66 (d, J = 1.8 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.08 (s, 1H), 8.24 (d, J = 5.7 Hz, 1H), 8.38 (s, 2H); 19F-NMR (282 MHz, CDCl3) δ = -62.9 (s, 6F); 13C-NMR (75 MHz, CD3OD) δ = 27.7, 37.9, 40.2, 98.3, 98.4, 117.4, 117.9, 121.5, 122.9, 124.7, 124.8, 125.1, 126.3, 126.4, 127.7, 128.7, 131.8 (q, JC-F = 126.0 Hz), 135.0, 136.7, 148.3, 151.0, 151.1, 151.2, 165.5.
N-(7-Chloro-4-quinolyl)-N’-(pentafluorobenzoyl)-1,3-diaminopropane, 48
Using 0.11 g (0.47 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and 2,3,4,5,6-pentafluorobenzoyl chloride (0.07 mL, 0.51 mmol) in the procedure described above and purification by flash chromatography with MeOH:CH2Cl2 (1:19 to 1:9 v/v), followed by extraction with 1N NaOH gave 0.13 g (0.3 mmol, 65% yield) of off-white crystals. 1H-NMR (300 MHz, CD3OD) δ = 2.03 (m, 2H), 3.46 (t, J = 7.2 Hz, 2H), 3.54 (t, J = 6.9 Hz, 2H), 6.53 (d, J = 5.4 Hz, 1H), 7.38 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.76 (d, J = 2.1 Hz, 1H), 8.08 (d, J = 9.0 Hz, 1H), 8.34 (d, J = 5.4 Hz, 1H); 19F-NMR (282 MHz, CD3OD) δ = -144.4 (m, 2F), -155.5 (tt, J = 2.5 Hz, J = 19.7 Hz, 1F), -164.0 (m, 2F); 13C-NMR (75 MHz, CD3OD) δ = 28.9, 38.7, 41.2, 99.5, 99.6, 113.4 (m), 118.7, 124.2, 126.0, 127.5, 127.6, 136.3, 138.9 (m), 143.3 (m), 145.0 (m), 149.5, 152.3, 152.5, 159.8.
N-(7-Chloro-4-quinolyl)-N’-(heptafluorobutyryl)-1,3-diaminopropane, 49
Using 0.11 g (0.47 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and perfluorobutyryl chloride (0.08 mL, 0.51 mmol) in the procedure described above and purification by flash chromatography with MeOH:CH2Cl2 (1:16 v/v), followed by extraction with 1N NaOH gave 0.1 g (0.23 mmol, 49% yield) of off-white crystals. 1H-NMR (300 MHz, CD3OD) δ = 1.98 (m, 2H), 3.39 (t, J = 7.2 Hz, 2H), 3.45 (t, J = 7.2 Hz, 2H), 6.50 (d, J = 5.7 Hz, 1H), 7.39 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.76 (d, J = 2.1 Hz, 1H), 8.06 (d, J = 9.0 Hz, 1H), 8.34 (d, J = 5.7 Hz, 1H); 19F-NMR (282 MHz, CD3OD) δ = -82.6 (t, J = 9.0 Hz, 3F), -122.2 (q, J = 9.0 Hz, 2F), -128.7 (s, 2F); 13C-NMR (75 MHz, CD3OD) δ = 28.6, 38.7, 41.1, 99.5, 99.6, 105.8-114.2 (m), 117.1 (t, JC-F = 141.0 Hz), 118.7, 120.9 (t, JC-F = 141.0 Hz), 124.1, 126.0, 126.0, 127.4, 127.5, 136.3, 149.4, 152.2, 152.5, 159.4 (t, JC-F = 95.9 Hz).
N-(7-Chloro-4-quinolyl)-N’-(pentadecafluorooctanoyl)-1,3-diaminopropane, 50
Employing 0.15 g (0.64 mmol) of N-(7-chloro-4-quinolyl)-1,3-diaminopropane and pentadecafluorooctanoyl chloride (0.17 mL, 0.7 mmol) in the procedure described above and purification by flash chromatography using MeOH:CH2Cl2 (1:19 to 1:9 v/v), followed by extraction with 1N NaOH gave 0.1 g (0.16 mmol, 25% yield) of off-white crystals. 1H-NMR (300 MHz, CD3OD) δ = 1.99 (m, 2H), 3.40 (t, J = 7.2 Hz, 2H), 3.45 (t, J = 6.9 Hz, 2H), 6.50 (d, J = 6.0 Hz, 1H), 7.39 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.77 (d, J = 2.1 Hz, 1H), 8.07 (d, J = 9.0 Hz, 1H), 8.34 (d, J = 6.0 Hz, 1H); 19F-NMR (282 MHz, CDCl3) δ = -78.8 (tt, J = 2.5 Hz, J = 9.9 Hz, 3F), -117.1 (t, J = 12.5 Hz, 2F), -118.9 (m, 2F), -119.4 (m, 2F), -120.1 (m, 2F), -123.7 (m, 2F); 13C-NMR (75 MHz, CD3OD) δ = 28.6, 38.8, 41.2, 106.8-117.2 (m), 118.7, 120.3 (t, JC-F = 112.8 Hz), 124.1, 126.0, 126.1, 127.5, 127.6, 136.3, 149.6, 152.3, 152.4, 159.5 (t, JC-F = 96.9 Hz).
Representative procedure for the synthesis of amide analogs 51-54
To a solution of N-(7-chloro-4-quinolyl)-N’-ethyl-N’-(2-aminoethyl)-1,2-diaminoethane 10 (0.031 g, 0.11 mmol) and Et3N (0.03 mL, 0.22 mmol) in anhydrous CH2Cl2, 3,5-bis(trifluoromethyl)benzoyl chloride (0.02 mL, 0.11 mmol) was added at 0 °C. The reaction mixture was stirred for 1 hour at room temperature until saturated 1N NaOH solution was added. The mixture was extracted with CH2Cl2, and the combined organic layers were dried over anhydrous Na2SO4, and concentrated in vacuo. Flash chromatography using MeOH:CH2Cl2 (1:99 v/v) as the mobile phase gave 0.051 g of the N-acyl quinolinium salt. The residue was refluxed in 5 mL of methanol for 6 hours and concentrated under reduced pressure. Flash chromatography using MeOH:CH2Cl2 (1:49 v/v) gave 0.022 g (0.04 mmol, 39% yield) of a yellow oil.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]-3,5-(bistrifluoromethyl)benzamide, 51
1H-NMR (300 MHz, CD3OD) δ = 1.13 (t, J = 7.2 Hz, 3H), 2.70-2.85 (m, 4H), 2.88 (t, J = 6.3 Hz, 2H), 3.40 (t, J = 6.0 Hz, 2H), 3.54 (t, J = 6.0 Hz, 2H), 6.49 (d, J = 5.7 Hz, 1H), 7.19 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.67 (d, J = 2.1 Hz, 1H), 7.82 (d, J = 9.0 Hz, 1H), 7.99 (s, 1H), 8.24 (s, 2H), 8.29 (d, J = 5.7 Hz, 1H); 19F-NMR (282 MHz, CDCl3) δ = -63.3 (s, 6F); 13C-NMR (75 MHz, CD3OD) δ = 12.2, 39.2, 41.4, 52.5, 53.4, 99.7, 99.8, 118.3, 119.0, 122.6, 123.7, 126.0 (m), 127.3, 127.4, 128.6, 129.8, 132.8 (q, JC-F = 126.0 Hz), 136.3, 137.7, 149.1, 152.2, 152.3, 166.4.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]pentafluorobenzamide, 52
Employing 0.047 g (0.16 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-N’-(2-aminoethyl)-1,2-diaminoethane 10 and 2,3,4,5,6-pentafluorobenzoyl chloride (0.023 mL, 0.16 mmol) in the procedure described above and purification by flash chromatography using MeOH:EtOAc (1:24 v/v) as the mobile phase gave 0.018 g (0.04 mmol, 23% yield) of light yellow crystals. 1H-NMR (300 MHz, CD3OD + CDCl3) δ = 1.13 (t, J = 6.9 Hz, 3H), 2.66-2.82 (m, 4H), 2.89 (t, J = 6.3 Hz, 2H), 3.38 (t, J = 5.7 Hz, 2H), 3.52 (t, J = 6.0 Hz, 2H), 6.43 (d, J = 5.7 Hz, 2H), 7.30 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.82 (d, J = 2.1 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 8.38 (d, J = 5.7 Hz, 1H); 19F-NMR (282 MHz, CDCl3) δ = -142.0 (m, 2F), -152.3 (dt, J = 3.1 Hz, J = 20.6 Hz, 1F), -161.4 (m, 2F); 13C-NMR (75 MHz, CD3OD) δ = 11.0, 37.6, 39.9, 47.3, 51.1, 51.8, 98.4, 98.5, 111.0 (m), 116.7, 121.6, 124.8, 126.5, 126.6, 135.1, 136.9 (m, JC-F = 930.6 Hz), 141.6 (m, JC-F = 958.8 Hz), 143.4 (m, JC-F = 958.8 Hz), 147.6, 150.3, 150.7, 157.8.
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]heptafluorobutanamide, 53
Using 0.047 g (0.16 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-N’-(2-aminoethyl)-1,2-diaminoethane 10 and perfluorobutyryl chloride (0.02 mL, 0.13 mmol) in the procedure described above and purification by flash chromatography with MeOH:EtOAc (1:24 v/v) gave 0.027 g (0.055 mmol, 42% yield) of a light yellow oil. 1H-NMR (300 MHz, CD3OD) δ = 1.06 (t, J = 7.2 Hz, 3H), 2.61-2.75 (m, 4H), 2.85 (t, J = 6.6 Hz, 2H), 3.39-3.49 (m, 4H), 6.56 (d, J = 5.7 Hz, 1H), 7.38 (dd, J = 2.1 Hz, J = 9.0 Hz, 1H), 7.78 (d, J = 2.1 Hz, 1H), 8.09 (d, J = 9.0 Hz, 1H), 8.36 (d, J = 5.7 Hz, 1H); 19F-NMR (282 MHz, CDCl3) δ = -82.6 (t, J = 9.0 Hz, 3F), -122.1 (q, J = 9.0 Hz, 2F), -128.7 (s, 2F); 13C-NMR (75 MHz, CD3OD) δ = 12.1, 39.0, 41.8, 48.8, 52.7, 53.5, 99.7, 99.8, 105.2-117.8 (m), 118.7, 120.8 (t, JC-F = 141.0 Hz), 124.2, 126.0, 127.5, 127.4, 136.4, 149.5, 152.3, 152.3, 152.6, 159.3 (t, JC-F = 94.0 Hz).
N-[2-{(N’-2-(7-chloro-4-quinolyl)aminoethyl-N”-ethyl}aminoethyl]pentadecafluorooctanamide, 54
Employing 0.051 g (0.17 mmol) of N-(7-chloro-4-quinolyl)-N’-ethyl-N’-(2-aminoethyl)-1,2-diaminoethane 10 and pentadecafluorooctanoyl chloride (0.04 mL, 0.17 mmol) in the procedure described above and purification by flash chromatography using MeOH:EtOAc (1:24 v/v) gave 0.019 g (0.027 mmol, 16% yield) of a light yellow oil. 1H-NMR (300 MHz, CD3OD) δ = 1.07 (t, J = 7.2 Hz, 3H), 2.61-2.76 (m, 4H), 2.87 (t, J = 6.6 Hz, 2H), 3.45 (q, J = 6.6 Hz, 1H), 6.59 (d, J = 6.0 Hz, 1H), 7.39 (dd, J = 2.4 Hz, J = 9.0 Hz, 1H), 7.79 (d, J = 2.4 Hz, 1H), 8.12 (d, J = 9.0 Hz, 1H), 8.37 (d, J = 5.7 Hz, 1H); 19F-NMR (282 MHz, CDCl3) δ = -82.7 (tt, J = 2.3 Hz, J = 9.9 Hz, 3F), -121.0 (t, J = 13.0 Hz, 2F), -122.9 (m, 2F), -123.4 (m, 2F), -123.9 (m, 2F), -124.1 (m, 2F), -127.6 (m, 2F); 13C-NMR (75 MHz, CD3OD) δ = 12.1, 39.0, 41.9, 52.6, 53.5, 99.7, 118.6, 124.3, 126.1, 127.1, 136.6, 149.0, 151.9, 152.9.
Acknowledgment
We thank the NIH (Grant RO1AI060792) for financial support.
References
- (1).De D, Byers LD, Krogstad DJ. J. Hetercyclic Chem. 1997;34:315–320. [Google Scholar]
- (2).O’Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. Pharmacol. Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- (3).Dominguez JN. Curr. Topics Med. Chem. 2002;2:1173–1185. doi: 10.2174/1568026023392986. [DOI] [PubMed] [Google Scholar]
- (4).Delarue S, Girault S, Maes L, Debreu-Fontaine MA, Labaeid M, Grellier P, Sergheraert C. J. Med. Chem. 2001;44:2827–2833. doi: 10.1021/jm010842o. [DOI] [PubMed] [Google Scholar]
- (5).O’Neill PM, Willock DJ, Hawley SR, Bray PG, Storr RC, Ward SA, Park BK. J. Med. Chem. 1997;40:437–448. doi: 10.1021/jm960370r. [DOI] [PubMed] [Google Scholar]
- (6).Madrid PB, Liou AP, DeRisi JL, Guy RK. P. falciparum. J. Med. Chem. 2006;49:4535–4543. doi: 10.1021/jm0600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Yearick K, Ekoue-Kovi K, Iwaniuk D, Natarajan JK, Alumasa J, de Dios AC, Roepe PD, Wolf C. J. Med. Chem. 2008;51:1995–1998. doi: 10.1021/jm800106u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- (9).Ridley RG. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- (10).Leed A, DuBay K, Ursos LM, Sears D, de Dios AC, Roepe PD. Biochemistry. 2002;41:10245–10255. doi: 10.1021/bi020195i. [DOI] [PubMed] [Google Scholar]
- (11).Chong CR, Sullivan DJ. Biochem Pharmacol. 2003;66:2201–2212. doi: 10.1016/j.bcp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- (12).de Dios AC, Casabianca LB, Kosar A, Roepe PD. Inorg Chem. 2004;43:8078–8084. doi: 10.1021/ic0489948. [DOI] [PubMed] [Google Scholar]
- (13).de Dios AC, Tycko R, Ursos LMB, Roepe PD. J. Phys. Chem. A. 2003;107:5821–5825. [Google Scholar]
- (14).Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden J. J. Med. Chem. 2000;43:283–291. doi: 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- (15).Cheruku SR, Maiti S, Dorn A, Scorneaux B, Bhattacharjee AK, Ellis WY, Vennerstrom JL. J. Med. Chem. 2003;46:3166–3169. doi: 10.1021/jm030038x. [DOI] [PubMed] [Google Scholar]
- (16).Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LMB, Sidhu ABS, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sidhu ABS, Verdier-Pinard D, Fidock DA. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. Mol. Microb. 2005;56:323–333. doi: 10.1111/j.1365-2958.2005.04556.x. [DOI] [PubMed] [Google Scholar]
- (19).Martin SK, Oduola AM, Milhous WK. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- (20).Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. J. Med. Chem. 2006;49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Weisman JL, Liou AP, Shelat AA, Cohen FE, Guy RK, DeRisi JL. Chem. Biol. Drug Des. 2006;67:409–416. doi: 10.1111/j.1747-0285.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Tang Y, Dong Y, Wittlin S, Charman SA, Chollet J, Chiu FC, Charman WN, Matile H, Urwyler H, Dorn A, Bajpai S, Wang X, Padmanilayam M, Karle JM, Brun R, Vennerstrom JL. Bioorg. Med. Chem. Lett. 2007;17:1260–1265. doi: 10.1016/j.bmcl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- (23).Dong Y, Tang Y, Chollet J, Matile H, Wittlin S, Charman SA, Charman WN, Tomas JS, Scheurer C, Snyder C, Scorneaux B, Bajpai S, Alexander SA, Wang X, Padmanilayam M, Cheruku SR, Brun R, Vennerstrom JL. Bioorg. Med. Chem. 2006;14:6368–6382. doi: 10.1016/j.bmc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- (24).Posner GH, Paik IH, Chang W, Borstnik K, Sinishtaj S, Rosenthal AS, Shapiro TA. J. Med. Chem. 2007;50:2516–2519. doi: 10.1021/jm070149m. [DOI] [PubMed] [Google Scholar]
- (25).Paik IH, Xie S, Shapiro TA, Labonte T, Narducci Sarjeant AA, Baege AC, Posner GH. J. Med. Chem. 2006;49:2731–2734. doi: 10.1021/jm058288w. [DOI] [PubMed] [Google Scholar]
- (26).Vennerstrom L, Arbe-Barnes S, Brun R, Charman SA, Chiu FC, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H, McIntosh K, Padmanilayam M, Santo Tomas J, Scheurer C, Scorneaux B, Tang Y, Urwyler H, Wittlin S, Charman WN. Nature. 2004;430(7002):900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- (27).O’Neill PM, Posner GH. J. Med. Chem. 2004;47:2945–2964. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- (28).Sachs J, Malaney P. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- (29).Ridley RG. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- (30).Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- (31).Riccio ES, Lee PS, Winegar RA, Krogstad DJ, De D, Mirsalis JC. Environ. Mol. Mutagen. 2001;38:69–79. doi: 10.1002/em.1052. [DOI] [PubMed] [Google Scholar]
- (32).Kaschula CH, Egan TJ, Hunter R, Basilico N, Parapani S, Tarameli D, Pasini E, Monti D. J. Med. Chem. 2002;45:3531–3539. doi: 10.1021/jm020858u. [DOI] [PubMed] [Google Scholar]
- (33).Ryckebusch A, Deprez-Poulain R, Debreu-Fontaine M-A, Vandaele R, Mouray E, Grellier P, Sergheraert C. Bioorg. Med. Chem. Lett. 2002;12:2595–2598. doi: 10.1016/s0960-894x(02)00475-4. [DOI] [PubMed] [Google Scholar]
- (34).Krungkrai J, Scozzafava A, Reungprapavut S, Krungkrai SR, Rattanajak R, Kamchongwongpaisan S, Supuran CT. Bioorg. Med. Chem. 2005;13:483–489. doi: 10.1016/j.bmc.2004.10.015. [DOI] [PubMed] [Google Scholar]
- (35).Klingenstein R, Melnyk P, Leliveld SR, Ryckebusch A, Korth C. J. Med. Chem. 2006;49:5300–5308. doi: 10.1021/jm0602763. [DOI] [PubMed] [Google Scholar]
- (36).Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam T.-g., Gray NS, Chatterjee A, Janes F, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. Proc. Nat. Acad. Sci. 2008;105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kim TW, Park J-H, Hong J-I. J. Chem. Soc., Perkin Trans. 2002:923–927. [Google Scholar]
- (38).Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Antimicrob. Agents Chemother. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Antimicrob. Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Antimicrob. Agents Chemother. 2007;51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).De D, Krogstad FM, Cogswell FB, Krogstad DJ. Am. J. Trop. Med. Hyg. 1996;55:579–583. doi: 10.4269/ajtmh.1996.55.579. [DOI] [PubMed] [Google Scholar]
- (42).Leon C, Rodriguez J, de Dominguez NG, Charris J, Gut J, Rosenthal PJ, Dominguez JN. Eur. J. Med. Chem. 2007;42:735–742. doi: 10.1016/j.ejmech.2007.01.001. [DOI] [PubMed] [Google Scholar]
- (43).Mahajan A, Yeh S, Nell M, van Rensburg CEJ, Chibale K. Bioorg. Med. Chem. Lett. 2007;17:5683–5685. doi: 10.1016/j.bmcl.2007.07.049. [DOI] [PubMed] [Google Scholar]
- (44).Chibale K, Moss JR, Blackie M, van Schalkwyk D, Smith PJ. Tetrahedron Lett. 2000;41:6231–6235. [Google Scholar]
- (45).Kaur K, Patel SR, Patil P, Jain M, Khan SI, Jacob MR, Ganesan S, Tekwani BL, Jain R. Bioorg. Med. Chem. 2007;15:915–930. doi: 10.1016/j.bmc.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Go M-L, Ngiam T-L, Wan ASC. J. Med. Chem. 1981;24:1471–1475. doi: 10.1021/jm00144a020. [DOI] [PubMed] [Google Scholar]
- (47).Delarue S, Girault S, Maes L, Debreu-Fontaine M-A, Labaeid M, Grellier P, Sergheraert C. J. Med. Chem. 2001;44:2827–2833. doi: 10.1021/jm010842o. [DOI] [PubMed] [Google Scholar]
- (48).Ryckebusch A, Deprez-Poulain R, Maes L, Debreu-Fontaine M-A, Mouray E, Grellier P, Sergheraert C. J. Med. Chem. 2003;46:542–557. doi: 10.1021/jm020960r. [DOI] [PubMed] [Google Scholar]
- (49).Musonda CC, Taylor D, Lehman J, Gut J, Rosenthal PJ, Chibale K. Bioorg. Med. Chem. 2004;14:3901–3905. doi: 10.1016/j.bmcl.2004.05.063. [DOI] [PubMed] [Google Scholar]
- (50).Ryckebusch A, Fruchart J-S, Cattiaux L, Rousselot-Paillet P, Leroux V, Melnyk O, Grellier P, Mouray E, Sergheraert C, Melnyk P. Bioorg. Med. Chem. Lett. 2004;14:4439–4443. doi: 10.1016/j.bmcl.2004.06.056. [DOI] [PubMed] [Google Scholar]
- (51).Musonda CC, Gut J, Rosenthal PJ, Yardley V, de Souza RCC, Chibale K. Bioorg. Med. Chem. 2006;14:5605–5615. doi: 10.1016/j.bmc.2006.04.035. [DOI] [PubMed] [Google Scholar]
- (52).Freitag M, Kaiser M, Larsen T, Zohrabi-Kalantari V, Heidler P, Link A. Bioorg. Med. Chem. 2007;15:2782–2788. doi: 10.1016/j.bmc.2006.12.034. [DOI] [PubMed] [Google Scholar]
- (53).Mei X, August AT, Wolf C. J. Org. Chem. 2006;71:142–149. doi: 10.1021/jo0518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wolf C, Liu S, Mei X, August AT, Casimir MD. J. Org. Chem. 2006;71:3270–3273. doi: 10.1021/jo060034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Liu S, Pestano JPC, Wolf C. Synthesis. 2007:3519–3527. doi: 10.1055/s-2007-990875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Natarajan JK, Alumasa J, Yearick K, Ekoue-Kovi KA, Casabianca LB, de Dios AC, Wolf C, Roepe PD. J. Med. Chem. 2008;51:3466–3479. doi: 10.1021/jm701478a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ansari AM, Craig JC. Synthesis. 1995:147–149. [Google Scholar]
- (58).Myers MC, Pokorski JK, Appella DH. Org. Lett. 2004;6:4699–4702. doi: 10.1021/ol0480980. [DOI] [PubMed] [Google Scholar]