Figure 6.
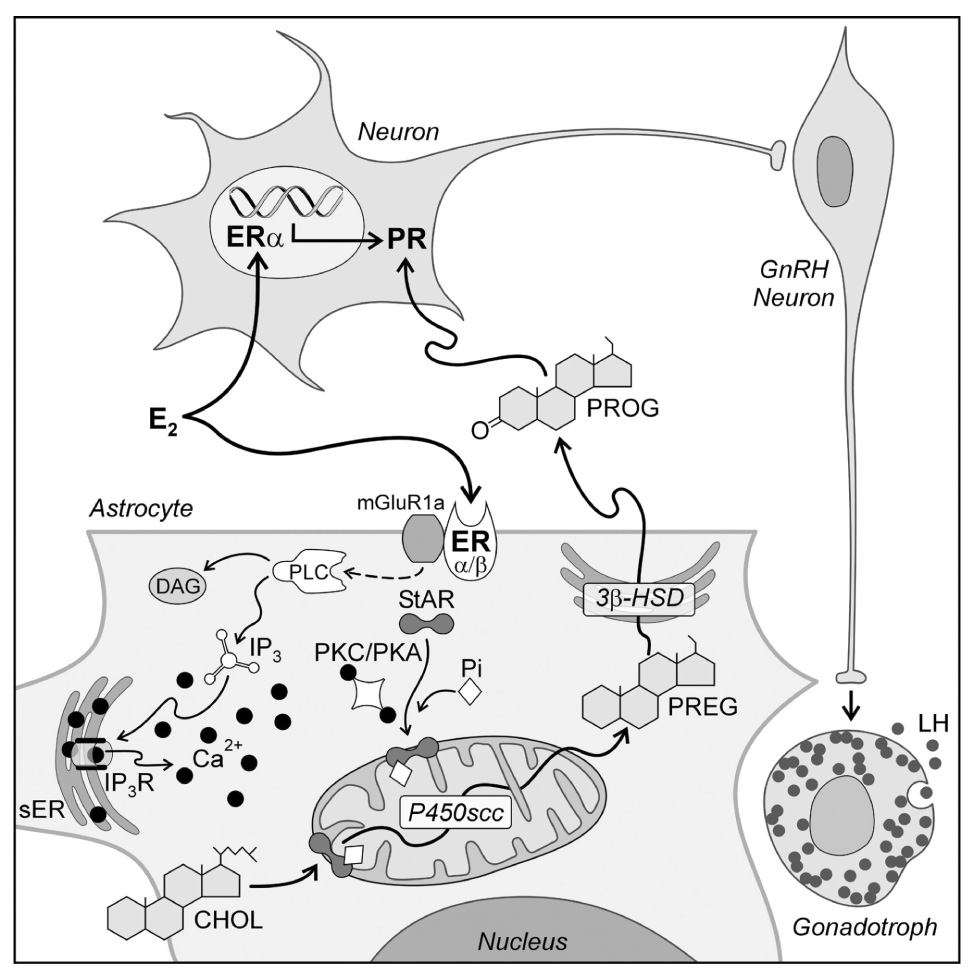
A model of estradiol action on hypothalamic cells involved in the regulation of the LH surge. Circulating estradiol acts on both neurons and astrocytes. The astrocyte has been expanded to illustrate the potential intracellular pathway of estradiol signaling that regulates progesterone (PROG) synthesis. Estradiol acts on membrane estrogen receptors (ER); both ERα and ERβ have been reported in astrocyte membranes (Chaban and Micevych, 2005). Activated ER increase [Ca2+]i by interacting with metabotropic glutamate receptor type 1a (mGluR1a) to initiate the phospholipase C (PLC)/ inositol triphosphate receptor (IP3R) mediated increase in [Ca2+]i. The source of the elevated [Ca2+]i is the smooth endoplasmic reticulum (sER). Estradiol-induced release of Ca2+ was mimicked with thapsigargin. Calcium may activate protein kinase A (PKA) and/or protein kinase C (PKC) phosphorylate StAR. StAR mediated transport of cholesterol (CHOL) through the mitochondrial intermembrane space is the rate limiting step of steroidogenesis. In the inner mitochondrial membrane, cholesterol (CHOL) is converted to pregnenolone (PREG) by cytochrome P450side chain cleavage enzyme (P450scc). PREG is converted to PROG in the sER via the action of 3β-hydroxysteroid dehydrogenase isomerase (3β-HSD) and diffuses out of the astrocyte to encounter estradiol induced PROG receptors (PR) in neurons. PR expressing neurons mediate the environmental and circadian signals to neurons stimulating GnRH. GnRH release in the median eminence in turn initiates the LH surge release from anterior pituitary gonadotrophs (modified from (Micevych et al., 2007).
