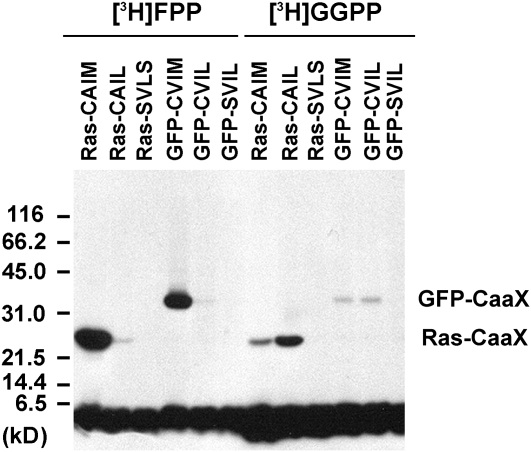
Figure 5.
In Vitro Isoprenylation of Modified GFP Fusion Proteins by BY-2 Cell-Free Extracts.
GFP fusion proteins were expressed in E. coli and tested for in vitro isoprenylation using cell-free extracts from 3-d-old BY-2 cells as a source of protein isoprenyltransferases and [3H]-FPP or [3H]-GGPP as isoprenyl diphosphate substrates. For comparison, Ras fusion proteins were also isoprenylated in vitro. Ras-CAIM is recognized by PFT, whereas Ras-CAIL is recognized by PGGT 1. By contrast, Ras-SVLS is not recognized by any known protein isoprenyltransferase. GFP-BD-CVIL was predicted to be a substrate of PGGT 1, whereas GFP-CVIM was predicted to be a substrate of PFT. GFP-SVIL served as a control protein that cannot be isoprenylated. The positions of GFP-CaaX and Ras-CaaX, which agree with the predicted molecular masses, are indicated. The broad band on the bottom of the gel corresponds to free radiolabeled substrate.