Figure 2.
RBP50, a 50-kD Poly(U) Binding Protein, Moves through the Translocation Stream and Has the Capacity to Bind, in a Sequence-Specific Manner, to Phloem-Mobile Transcripts.
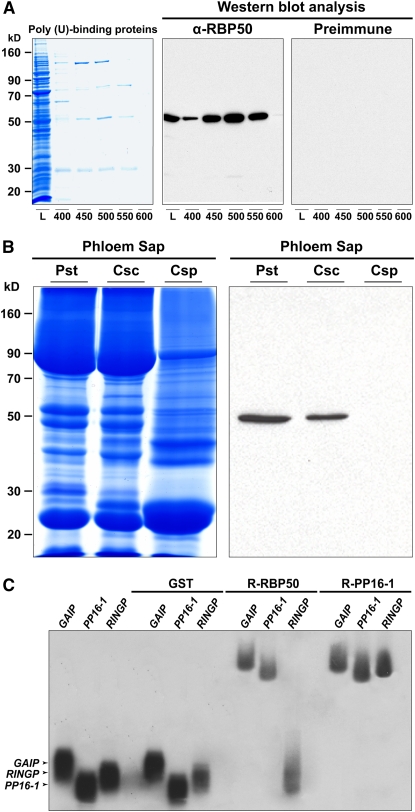
(A) A poly(U)-affinity column was used to purify pumpkin phloem proteins with poly(U) binding capacity. Candidate proteins were eluted using the following step concentrations of NaCl: 400, 450, 500, 550, and 600 mM. Protein profiles (L; loading sample) were separated on a 12% SDS-PAGE gel (volumes of 20 μL were applied for each fraction) and then visualized with GBS reagent. Purified anti-RBP50 antibody (α-RBP50) specifically detected the 50-kD protein (middle panel), whereas preimmune sera did not cross-react with any protein, including those in the loading sample.
(B) Long-distance movement of RBP50 is confirmed by heterografting studies. Phloem sap was collected from pumpkin stock (Pst), cucumber scions grafted onto pumpkin stocks (Csc), and wild-type cucumber plant (Csp). Phloem sap proteins were resolved by SDS-PAGE (left panel) and subjected to protein gel blot analysis with anti-RBP50 antibody (right panel). Detection of RBP50 in phloem sap collected from cucumber scions establishes that this protein is phloem-mobile.
(C) Gel mobility-shift assays reveal that recombinant (R) RBP50 binds to phloem-mobile transcripts in a sequence-specific manner. RNA binding capacities of RBP50, PP16-1, and GST were tested against 32P-labeled in vitro–transcribed riboprobes for GAIP, PP16-1, and RINGP. Note that GST did not bind to these transcripts, RBP50 bound to GAIP and PP16-1 but not RINGP, and PP16-1 bound all three probes.