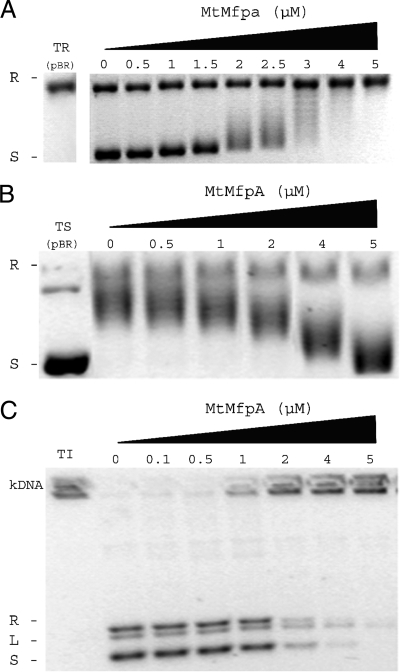
FIG. 1.
MfpAMt inhibits the catalytic activity of M. tuberculosis DNA gyrase in topoisomerase assays. (A) Concentration-dependent inhibitory effect of MfpAMt on supercoiling activity of M. tuberculosis DNA gyrase. Supercoiling assays were performed using relaxed pBR322 as the substrate (TR) and final concentrations of MfpAMt (μM) as indicated. R, relaxed pBR322; S, supercoiled pBR322. (B) Concentration-dependent inhibitory effect of MfpAMt on ATP-independent relaxation of supercoiled DNA. Supercoiled pBR322 (TS) was the substrate. (C) Concentration-dependent inhibitory effect of MfpAMt on decatenation activity of M. tuberculosis DNA gyrase. Interlinked kDNA (kDNA) was used as the substrate (TI). Decatenated minicircles were visualized in three forms: relaxed (R), linearized (L), and supercoiled (S).