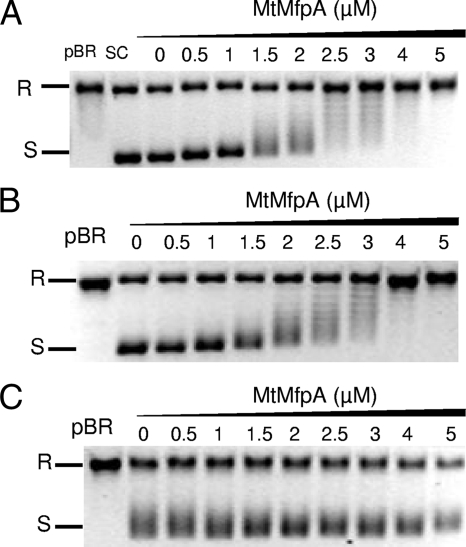
FIG. 4.
The MfpAMt effect on DNA gyrase catalysis is abolished with M. tuberculosis DNA gyrase harboring the D87G but not the A83S substitution of GyrA. (A) Supercoiling assays were performed with wild-type M. tuberculosis gyrase and relaxed pBR322 as a substrate. R, relaxed pBR322; S, supercoiled pBR322. Reactions were carried out without MfpAMt (lane 0) and with increasing concentrations of MfpAMt (final concentrations in μM are indicated above the gel). MfpAMt inhibited gyrase supercoiling in a concentration-dependent manner. (B) Same experiment with an altered M. tuberculosis DNA gyrase composed of GyrA A83S and wild-type GyrB subunit, which efficiently supercoiled pBR322 (lane 0). MfpAMt inhibited altered gyrase supercoiling in a concentration-dependent manner, as for the wild-type gyrase. (C) Same experiment with an altered M. tuberculosis DNA gyrase composed of GyrA D87G and the wild-type GyrB subunit, which efficiently supercoiled pBR322 (lane 0). No modification of this supercoiling activity in the presence of MfpAMt (from 0.5 to 5 μM) was observed.