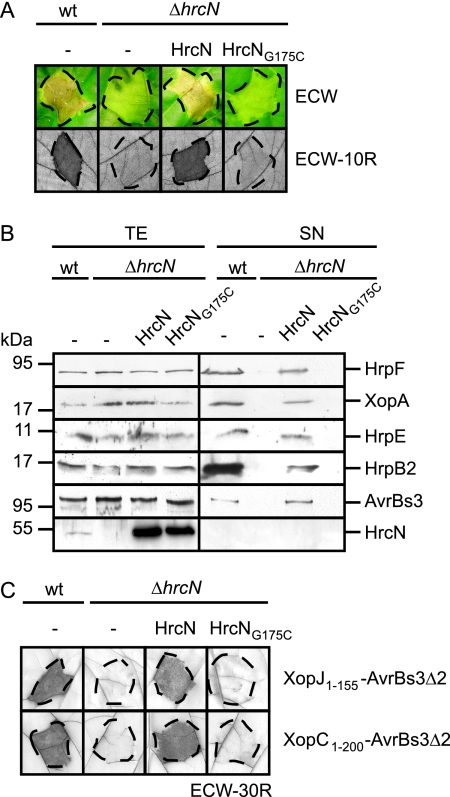
FIG. 1.
The predicted ATPase HrcN is essential for T3S and bacterial pathogenicity. (A) HrcN is essential for the interaction of X. campestris pv. vesicatoria with the plant. X. campestris pv. vesicatoria strains 85-10 (wild type [wt]) and 85-10ΔhrcN (ΔhrcN) carrying the empty vector (−) or expressing Strep-hrcN (HrcN) and Strep-hrcNG175C (HrcNG175C) as indicated were inoculated into susceptible ECW and resistant ECW-10R pepper plants. Disease symptoms were photographed 7 days after inoculation. For better visualization of the HR in ECW-10R plants, leaves were bleached in ethanol 3 days after inoculation. Dashed lines indicate the inoculated areas. (B) HrcN is crucial for efficient in vitro T3S. Strain 85* (wt) and 85*ΔhrcN (ΔhrcN) carrying the empty vector (−) or synthesizing Strep-HrcN or Strep-HrcNG175C as indicated were incubated in secretion medium. Total protein extracts (TE) and culture supernatants (SN) were analyzed by immunoblotting, using HrpF-, XopA-, HrpE-, HrpB2-, AvrBs3-, and HrcN-specific antibodies. HrpF, XopA, HrpE, and HrcN were analyzed in strains 85* and 85*ΔhrcN. For the analysis of AvrBs3 secretion, we used strains 82* and 82*ΔhrcN, which naturally deliver AvrBs3. Note that HrpB2 secretion was analyzed in the hpaC deletion mutant strains 85*ΔhpaC and 85*ΔhrcNΔhpaC, since HrpB2 secretion in hpaC wild-type strains is at the detection limit of the HrpB2-specific antibody (42). (C) HrcN is essential for translocation of XopJ and XopC. XopJ1-155-AvrBs3Δ2 and XopC1-200-AvrBs3Δ2 were synthesized in strains 85-10 and 85-10ΔhrcN carrying the empty vector (−) or the Strep-hrcN or Strep-hrcNG175C expression construct as indicated. Bacteria were inoculated into AvrBs3-responsive ECW-30R pepper plants. Three days after inoculation, leaves were bleached in ethanol to better visualize the HR. Dashed lines indicate the inoculated areas.