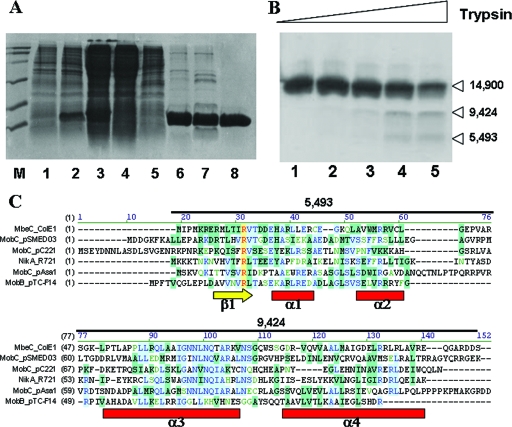
FIG. 2.
(A) Purification of MbeC-His6. Fractions from the different purification steps were analyzed by SDS-12% PAGE. Lane M, molecular weight marker; lane 1, BL21(DE3)/pUIV239 before induction; lane 2, BL21(DE3)/pUIV239 after 4 h induction; lane 3, clear lysate; lane 4, flowthrough from affinity chromatography; lane 5, wash from affinity chromatography; lane 6, 6 μg after affinity chromatography; lane 7, 5 μg of sample after ion-exchange chromatography; lane 8, 3 μg of sample after gel filtration chromatography. (B) Limited trypsin digestion of MbeC-His6. The results are visualized by SDS-15% PAGE. Lane 1, MbeC-His6 without trypsin. Lanes 2, 3, 4, and 5 show results for 0.5, 1.5, 5, and 15 μM trypsin, respectively. Molecular masses (in daltons) of the obtained fragments as determined by MALDI-TOF mass spectrometry are indicated on the right. (C) CLUSTAL W alignment of full-length ColE1 MbeC with other representative MOBHEN and MOBP accessory proteins. Secondary structure prediction (Jpred, PSIPRED, and GOR4) is shown below the alignment, and the length and mass of trypsin-produced fragments is shown above the alignment.