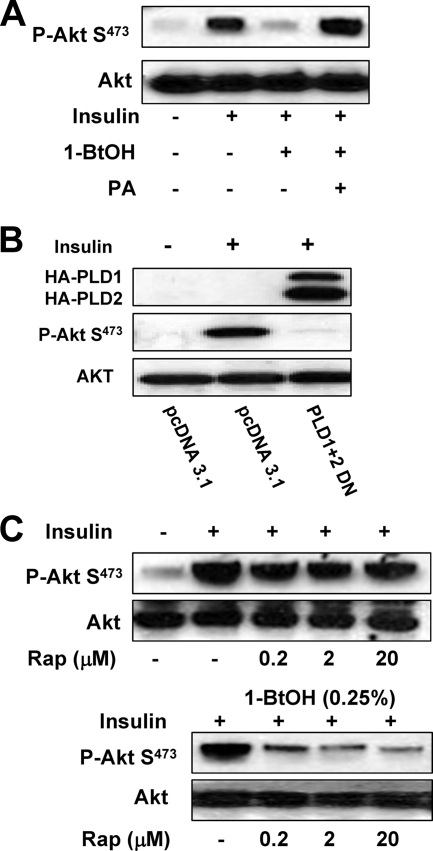
FIG. 6.
Insulin-stimulated Akt phosphorylation at Ser473 is dependent on PLD activity. (A) MDA-MB-231 cells were plated at 5 × 105/60-mm plate in medium containing 10% serum and incubated for 24 h. Cells were then shifted to medium containing 0.5% serum overnight. Insulin (100 nM), 1-BtOH (0.8%), and PA (100 μM) were then added as indicated. The cells were harvested 2 h later, and the levels of Akt phosphorylated at Ser473 (P-Akt S473) and Akt were determined by Western blot analysis. (B) MDA-MB-231 cells were plated at 5 × 105/60-mm plate as in Fig. 2. Twenty-four hours later, the cells were transfected with vectors expressing the catalytically inactive dominant negative (DN) mutant forms of PLD1 and PLD2 or the parental vector pcDNA3.1 as indicated. Twenty-four hours later, the cells were treated with fresh medium containing 10% serum for an additional 24 h. The cells were then harvested and analyzed for the levels of Akt phosphorylated at Ser473 (P-Akt S473) and Akt as in panel A. (C) MDA-MB-231 cells were plated as in panel A. Rapamycin (Rap) was added at the indicated concentrations in the absence or presence of 0.25% 1-BtOH as indicated. Six hours later, the cells were harvested and the levels of phosphorylated Akt and Akt were determined as in panel A. The data shown are representative of two independent experiments.