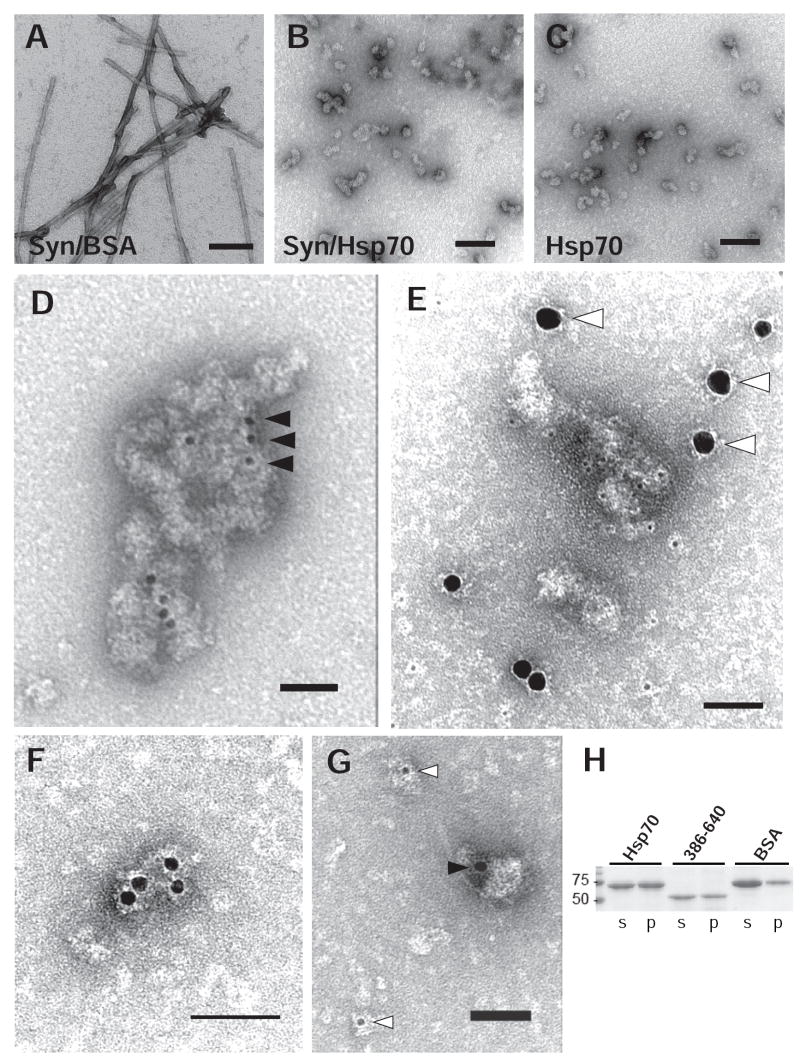
FIG. 4. Characterization of α-Syn in the presence of Hsp70 by EM.
Fibril assembly reactions containing full-length α-Syn (72 μM) were incubated for 96h in the presence of either 7.2 μM BSA (A) or Hsp70 (B). Reactions containing Hsp70 alone were also included (C). Three μL of each reaction was adsorbed onto carbon/formvar-coated grids and visualized by transmission EM following negative stain with 1% uranyl acetate. In contrast to uniform fibrils 10-14 nm in caliber, addition of Hsp70 resulted in amorphous objects ranging from 20-50 nm and larger aggregates. Samples from α-Syn assembly reactions containing Hsp70 were immunostained using antibodies against Hsp70 (3a3, black arrowheads) and α-Syn (SNL-1, white arrowheads). Single labeling with anti-Hsp70 antibody revealed strong staining of amorphous aggregates (D). In contrast, double labeling did not show colocalization of anti-α-Syn with either aggregates or Hsp70 (E,F,G). Scale bars: 500nm (A,B,C); 100nm (D,E); 50nm (F,G). H, Hsp70, GST-Hsp70386-640, or BSA were incubated in the absence of α-Syn for 72 h with agitation and separated by SDS-PAGE following centrifugation (100,000 × g). Coomassie Blue staining of proteins shows that Hsp70 and its substrate binding alone are capable of forming pelletable aggregates.