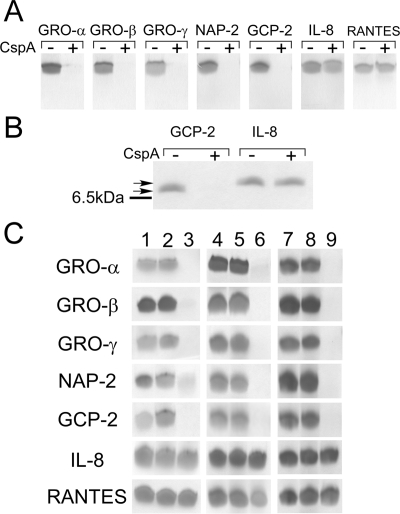
FIG. 3.
Chemokine cleavage by CspA. Chemokines were incubated with purified CspA, whole GBS cells, or media from cspA-expressing L. lactis cultures. The reaction products were then analyzed by Tris-Tricine-SDS-PAGE. (A) Chemokine cleavage by purified CspA. Two micrograms of each recombinant chemokine (Peprotech) was incubated for 1 h at 37°C with PBS (−) or 13.7 U of CspA (+). Reaction products were analyzed using Tris-Tricine-SDS-PAGE (13.5% acrylamide). (B) The same experiment described in the legend to panel A was performed, except that a 20% acrylamide-Tris-Tricine gel system was utilized. The line to the left of the gel indicates the migration position of the bovine aprotinin standard (6.5 kDa), the lower arrow indicates the migration position of mock-treated GCP-2 (7.9 kDa), and the upper arrow indicates the migration position of mock-treated IL-8 (8.4 kDa). (C) Chemokine cleavage by wt CspA and catalytic site variants of CspA. Lanes 1 to 3, cleavage of chemokines by whole GBS cells. Chemokines were untreated (lane 1) or incubated with logarithmic-phase (OD600 = 0.3), concentrated, PBS-washed whole cells of GBS strain DS107 (expressing S575A CspA) (lane 2) or with identically prepared whole cells of wt GBS strain COH1 (lane 3). Lanes 4 to 9, chemokine cleavage by medium supernatants from lactococcal strains expressing the D180A and S575A CspA variants. Chemokines were incubated for 1 h at 37°C with PBS (lanes 4 and 7) or with equivalent volumes of concentrated L. lactis medium supernatants from strains expressing D180A CspA (lane 5), S575A CspA (lane 8), or wt CspA (lanes 6 and 9).