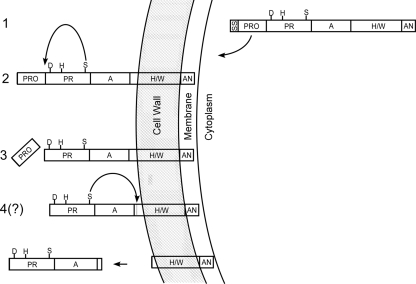
FIG. 5.
Model for CspA export, maturation, and processing. Depicted are the cell wall, membrane, and cytoplasm of GBS. Putative assignment and nomenclature of CspA domains were predicted in silico in conjunction with comparisons of other CEPs by Siezen (24). The predicted SS and PRO domain and the protease domain (PR) with residues (S575, D180, and H246 [S, D, and H]) constituting the putative serine protease catalytic site are indicated, as are the domains A (domain of unknown function), H/W (helical/cell wall-spanning region), and AN (cell wall anchor bearing the LPXTG motif). After the synthesis of the immature protein (1), the SS is recognized by the host, resulting in protein translocation across the membrane and SS removal. Subsequently, the protein is covalently anchored to the cell wall (2) via the AN domain, presumably by a host sortase-dependent mechanism (16), and CspA catalyzes the removal (arrow) of its N-terminal PRO domain via its serine active site (3). Finally, evidence from this and previous work (23) suggests that recombinant CspA autocatalyzes its own C-terminal cleavage (arrow), which may allow the release of the protease from the cell (4).