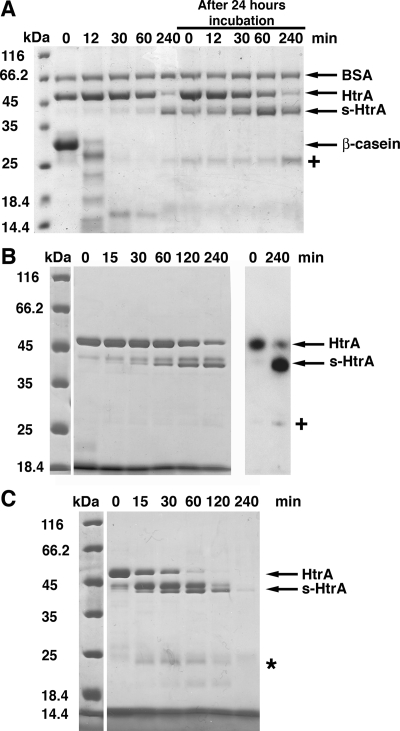
FIG. 4.
Degradation peptides products stimulate HtrA autocleavage. (A) A proteolytic reaction containing β-casein (66 μM) in the presence of HtrA (4.1 μM) was incubated for 4 h at 37°C. Samples were obtained at the indicated times, resolved by SDS-PAGE, and stained with Coomassie brilliant blue. After the reaction was kept at 37°C for 24 h, fresh HtrA (4.1 μM) was added, and the incubation was continued for four additional hours. Samples were taken at the indicated times to monitor the accumulation of s-HtrA protein. BSA protein (2 μg) was added to each sample as a loading control. (B) β-Casein peptides produced by HtrA hydrolysis were incubated with HtrA (4.1 μM) for 4 h at 37°C. At the indicated time points, samples were obtained and resolved by SDS-PAGE (left). The samples at time zero and 240 min were also processed for Western blotting with a rabbit anti-HtrA serum (right). The “+” sign indicates the 25-kDa self-cleavage form of HtrA recognized by the anti-HtrA serum. (C) β-Casein peptides produced by trypsin hydrolysis were mixed with HtrA (4.1 μM) in an autocleavage reaction for 4 h at 37°C. Time points of the reaction were obtained and resolved by SDS-PAGE. The asterisk indicates small peptides produced from HtrA and s-HtrA proteins by a residual amount of trypsin incorporated in the autocleavage reaction along with the β-casein peptides.