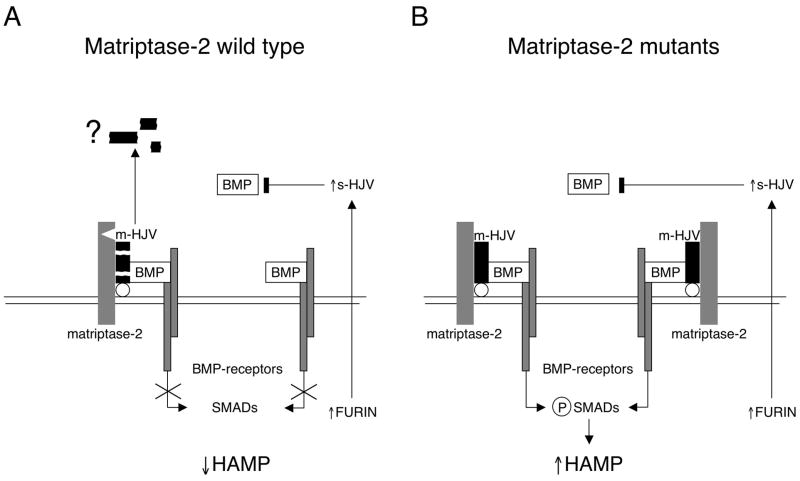
Figure 7. Model of matriptase-2 activity on BMP-HJV-hepcidin pathway.
(A) Schematic representation of a model of matriptase-2 activity in iron deficiency. On the left, the serine protease cleaves m-HJV releasing soluble fragments (here simplified by the black boxes). The cleavage sites of matriptase-2 are unknown. The question mark indicates uncertainty on fragments function. The resulting hepcidin inhibition is shown. The complementary effect of s-HJV, produced by furin cleavage, to sequester BMP is shown on the right. (B) Lack of hepcidin inhibition in the presence of matriptase-2 mutations. m-HJV acts as BMP coreceptor and permits hepcidin production in iron deficiency; the effect of s-HJV cannot downregulate hepcidin in the presence of m-HJV.