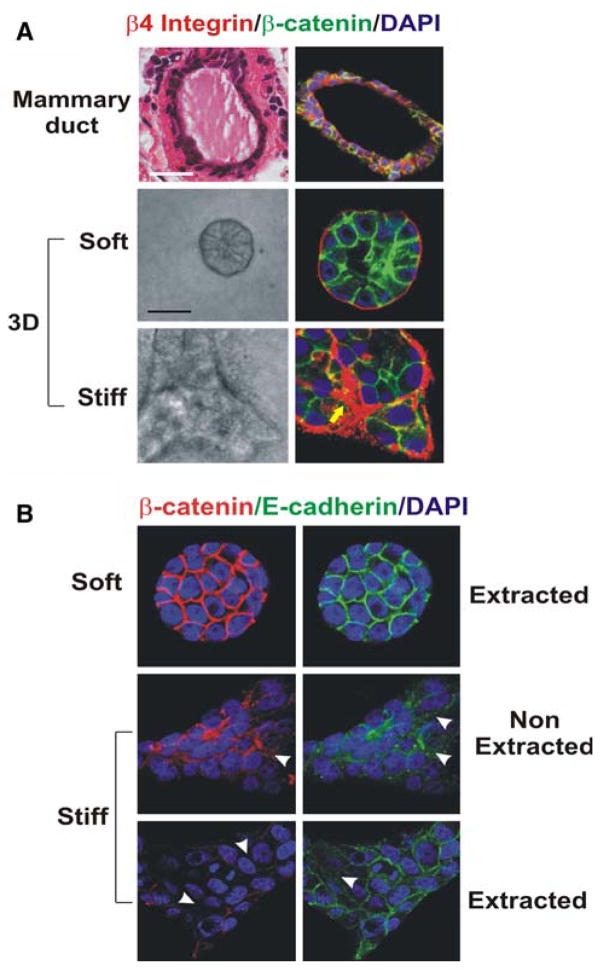
Fig. 1.
Mammary epithelial growth and morphogenesis is regulated by matrix stiffness. (a) 3D cultures of normal mammary epithelial cells (MECs) within collagen gels of different concentration. Stiffening the ECM through an incremental increase in collagen concentration (soft gels: 1 mg/ml Collagen I, 140 Pa; stiff gels 3.6 mg/ml Collagen I, 1,200 Pa) results in the progressive perturbation of morphogenesis, and the increased growth and modulated survival of MECs. Altered mammary acini morphology is illustrated by the destabilization of cell–cell adherens junctions and disruption of basal tissue polarity indicated by the gradual loss of cell–cell localized β-catenin (green) and disorganized β4 integrin (red) visualized through immunofluorescence and confocal imaging. (b) Confocal immunofluorescence images of MEC colonies on soft and stiff gels (140 vs. >5,000 Pa) stained for β-catenin (red) and E-cadherin (green), and counterstained with DAPI (blue) after triton X-100 extraction. β-catenin could be extracted from the sites of cellcell interaction in MEC colonies formed on a stiff but not on a soft gel, indicating that adherens junctions are less stable in MEC structures formed on stiff gels. White arrows indicate diffuse staining patterns of β-catenin and E-cadherin (Modified from Kass et al. [18])