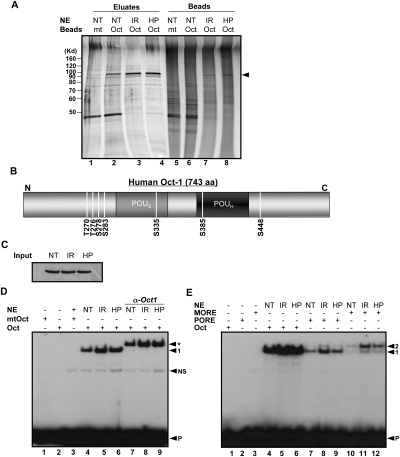
Figure 1.
Genotoxic and oxidative stress induce Oct1 phosphorylation and dimerization at complex sites. (A) Oct1 was purified from HeLa nuclear extracts using nanoparticles coupled to multimerized octamer or mutant sequences. HeLa cells were treated with IR or H2O2 and incubated for 1 h. The eluted proteins were resolved using SDS-PAGE and silver stained. (NT) No treatment; (HP) H2O2. (Arrow) Oct1 band. (B) Identified Oct1 serine and threonine phosphorylation events superimposed on a schematic of the Oct1 amino acid structure. DNA-binding domain modification events identified separately by the Gygi laboratory are also shown. (C) Oct1 Western blot showing protein levels in the input HeLa nuclear extracts. (D) EMSA using a simple octamer probe and extracts from C. Arrows indicate free probe (P), nonspecific band (NS), monomeric Oct1 (1), and Oct1 antibody supershifted band (*). (E) EMSA using radiolabeled simple octamer, PORE, and MORE probes. Arrows indicate monomeric (1) and dimeric (2) occupancy, and free probe (P).