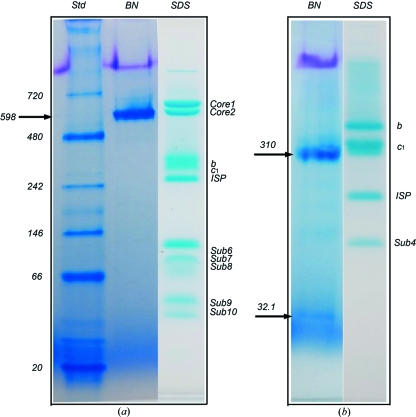
Figure 1.
BN–PAGE analysis of membrane proteins with known crystal structures. (a) BN–PAGE and tricine–SDS–PAGE of the 11-subunit bovine heart mitochondrial cytochrome bc 1 complex. Two PAGE gels are shown; the left two lanes are results from a BN–PAGE run and are as labeled; the right lane shows the result from tricine–SDS–PAGE (Schagger, 2006 ▶) of the same sample, which gives rise to at least ten bands for the intact bovine bc 1 complex. Purified bovine bc 1 in a buffer (50 mM Tris–HCl pH 8.0 supplemented with 0.66 M sucrose) was diluted to a final concentration of 1 mg ml−1 using the same buffer before a BN–PAGE run. There was no need for additional detergents in the dilution because the purified bovine bc 1 contained a sufficient amount of potassium deoxycholate. The arrow indicates the position of the bovine bc 1 complex in BN–PAGE. (b) BN–PAGE and tricine–SDS–PAGE of the four-subunit bacterial cytochrome bc 1 complex from R. sphaeroides. The Rsbc 1 sample in a buffer (50 mM Tris–HCl pH 8.0, 200 mM NaCl, 200 mM histidine, 0.5% β-OG) at a concentration of 1 mg ml−1 was used for BN–PAGE. Two bands were shown by the BN–PAGE as indicated by arrows; the top arrow indicates the position of the three-subunit Rsbc 1 complex dimer and the lower arrow indicates the position of the dissociated subunit 4. Four subunits are shown for the same sample by the tricine–SDS–PAGE as indicated.