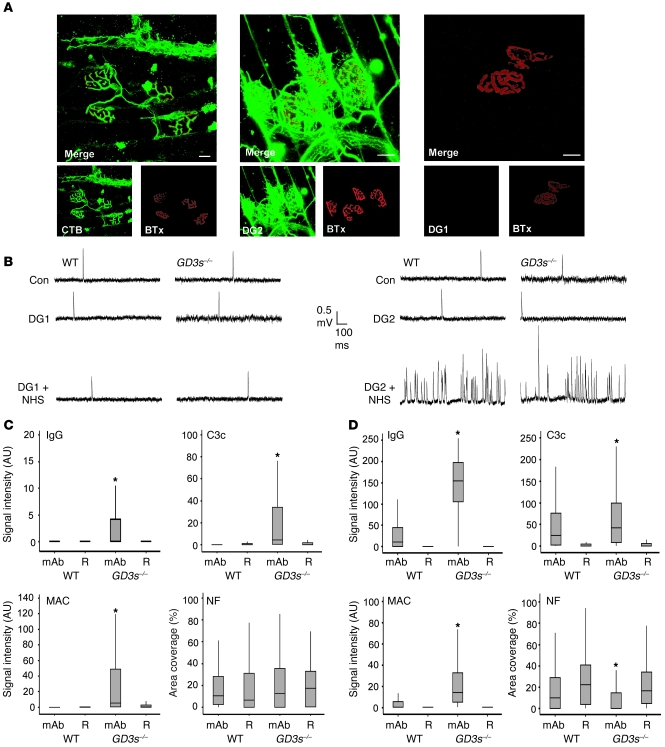
Figure 2. Pathophysiological effects of anti-GM1 antibodies in living nerve-muscle preparations ex vivo.
(A) Reconstructed confocal images of triangularis sterni NMJs from GD3s–/– mice, following ex vivo incubation in CTB, DG2, and DG1. For CTB and DG2, axonal staining is present along with staining of the parajunctional fibroblast. DG1 binding is undetectable. (B) Electrophysiology in ex vivo hemidiaphragm of WT and GD3s–/– mice. mAbs were applied, followed by a source of complement, and under such conditions only DG2 caused an increase in MEPP frequency of each strain. Con, control. (C) DG1 effect in ex vivo hemidiaphragm (WT and GD3s–/–). Following ex vivo muscle nerve incubations in mAb (or Ringer’ s medium alone [R] as control), tissue was snap-frozen and sectioned for quantitative analysis. Graphs show IgG, C3c, and MAC deposition (quantified as the signal intensity over the NMJ). Neurofilament (NF) loss is quantified as area coverage over the NMJ. DG1 binding is undetectable in the WT NMJ and only weakly present in the GD3s–/– NMJ under maximal detection settings. MAC and C3c deposition do not translate into neurofilament loss. (D) The experiment was performed as described for C, but with the mAb DG2. DG2 is detectable in WT and GD3s–/– NMJs and activates complement to cause a neurofilament loss compared with Ringer’s medium alone–incubated control tissue. For both C and D, note that the y axes have been adjusted for clarity. *P < 0.05 compared with the Ringer’s control. Scale bars: 20 μm.