Figure 1.
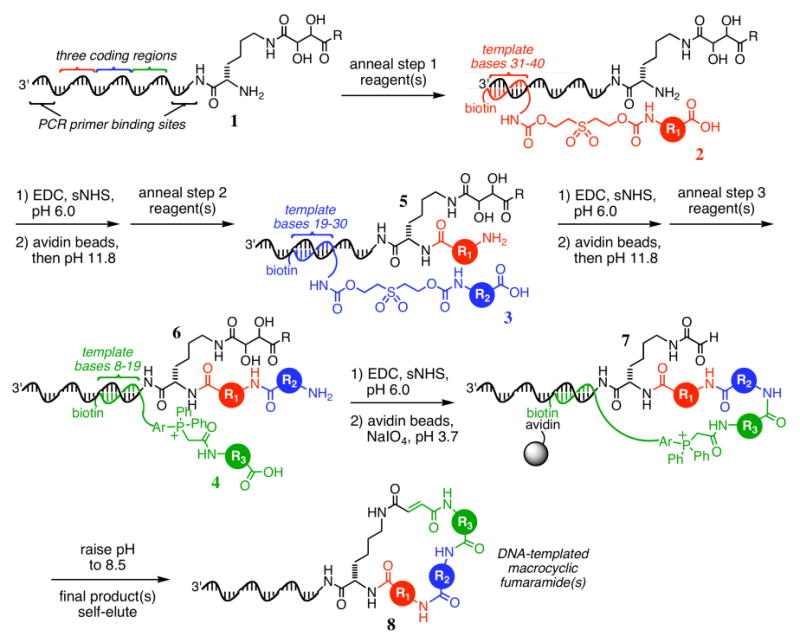
The previously reported15 scheme for the synthesis of a DNA-templated macrocycle library, where R is −NHCH3 or tryptamine, and Ar is −(p-C6H4)–. In contrast with the capping-based method described in the present work, each DNA-templated reaction in this scheme requires a bond formation step, a streptavidin bead capture step, a washing step, and a product elution step.
