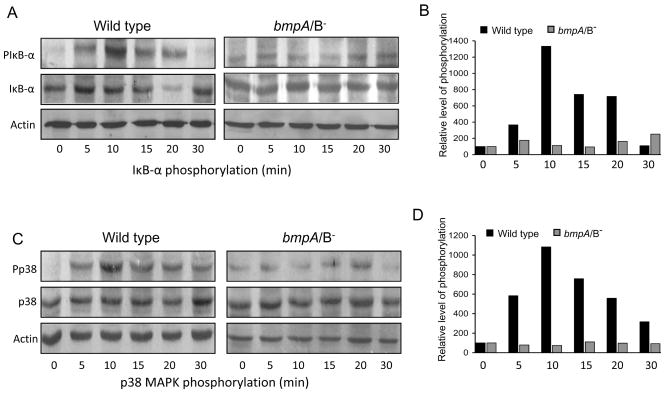
Fig. 4.
bmpA/B mutant B. burgdorferi has impaired ability to induce phosphorylation of IκB-α and p38. (A) Phosphorylation of IκB-α. Cells were exposed to wild type and bmpA/B mutant spirochetes and harvested at 0, 5, 10, 15, 20 and 30 min following B. burgdorferi exposure. Western blot analysis was performed on whole cell lysates using antibodies that specifically recognize phosphorylated (PIκB-α) and total (IκB-α) forms of IκB-α proteins. Cellular actin level was used as a loading control. A representative of three experiments is presented. (B) Densitometric analysis of IκB-α phosphorylation. Relative densities of phosphorylated and total IκB-α levels in synovial cells exposed to wild type (black bar) and bmpA/B mutant B. burgdorferi (gray bar) were determined by densitometric scan. Data represented as the relative ratio of phosphorylated and total IκB-α levels, and the values of 0 minute time points, were considered as 100%. (C) and (D) Phosphorylation of p38 MAPK. The cell lysates were assessed by Western blotting using antibodies that specifically recognize phosphorylated (Pp38) and total (p38) forms of p38 MAPK proteins, followed by densitometric analysis with similar parameters as described for IκB-α in (A) and (B) above.