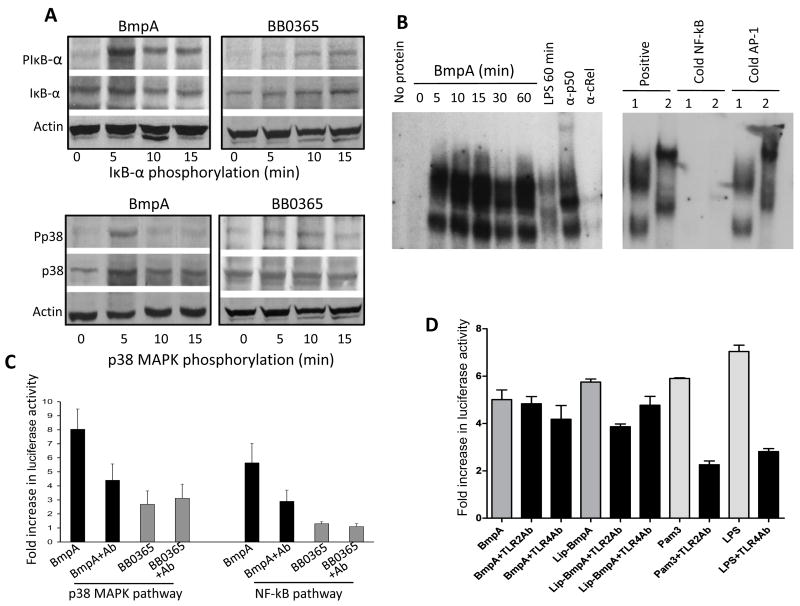
Fig. 5.
Non-lipidated BmpA activates NF-κB or p38 MAPK signaling pathways in synovial cells. (A) Phosphorylation of IκB-α and p38 MAPK. Cells were harvested at indicated times after treatment with non-lipidated BmpA or BB0365 (300 nanogram/ml), and phosphorylation of IκB-α was assessed by Western blotting using antibodies that specifically recognize phosphorylated (PIκB-α) and total (IκB-α) forms of IκB-α proteins (upper panels). Lower panels indicate phosphorylation of p38 MAPK assessed using antibodies that specifically recognize phosphorylated (Pp38) and total (p38) forms of p38 MAPK proteins. (B) BmpA-mediated induction NF-κB was assessed by electromobility shift assays (EMSA). The left panel shows a time course of nuclear extracts binding to an oligonucleotide probe containing consensus NF-κB binding sites. Synovial cells were stimulated for the indicated times with BmpA or LPS (control). The panel also shows a representative super shift assay using antibodies specific for p50 and c-Rel. The reactions using BmpA- (1) or LPS- (2) induced nuclear extracts were performed in the absence or presence of excess (100X) unlabeled oligonucleotides (cold NF-κB, right panel). The competition reactions were also carried out with cold AP-1 consensus binding sequences (negative control). (C) Activation of NF-κB or p38 MAPK signal transduction pathways in transfected synovial cells. Cells were co-transfected with phRL-TK plasmid (internal control) and pNF-κB-Luc (NF-κB reporter) or pFR-Luc and pFA-CHOP (p38 reporter). Transfected cells were exposed to 300 nanogram/ml of either non-lipidated recombinant proteins, BmpA (black bar) or control B. burgdorferi protein, BB0365 (gray bar) in the presence or absence of BmpA antibodies (1: 100 dilution). After 18 hours of incubation, luciferase activities were determined in each sample, and differential transfection efficiencies were normalized using Renilla luciferase activities. Specific luciferase activities of each group were presented as fold increase compared to the corresponding zero time values. Bars represent the mean ± standard error from three independent experiments. Luciferase reporter gene expression in BmpA exposed cells is significantly higher than that of BB0365 treated cells (P< 0.001), which is abrogated by the addition of BmpA antibody (P< 0.01). (D) TLR antibody-mediated inhibition of BmpA-induced activation of NF-κB signal transduction pathways in transfected cells. Synovial cells were co-transfected with phRL-TK plasmid (internal control) and pNF-κB-Luc (NF-κB reporter) plasmids, exposed to either 300 nanogram/ml of recombinant BmpA proteins (dark gray bars) or known TLR2 or TLR4 agonists, Pam3CSK4 or LPS, respectively (light gray bars). In addition, selected cells were also incubated with either TLR2 or TLR4 antibodies (black bars). After 18 hr of incubation, luciferase activities were determined and presented as fold increase relative to the corresponding zero time values. Bars represent the mean ± standard error from three independent experiments. TLR2 antibody significantly reduced the luciferase expression in cells exposed to either lipidated BmpA or Pam3CSK4 (P<0.004), but did not alter the reporter gene expression in cells exposed to non-lipidated BmpA (P>0.05). TLR4 antibody, while reduced the LPS-mediated luciferase expression (P<0.005), did not influence either lipidated or non-lipidated BmpA-mediated reporter gene expression (P>0.05).