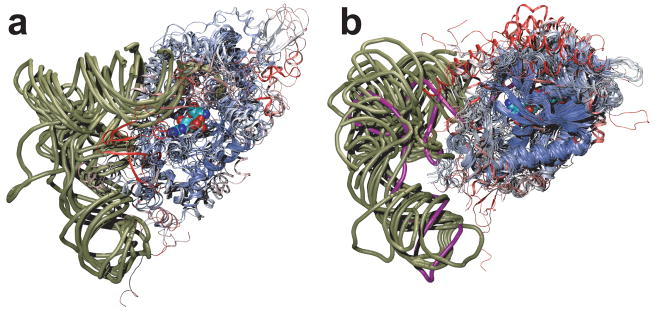
Figure 2.
Class I (a) and class II (b) tRNA synthetase:tRNA complexes are structurally aligned. Only a single monomer of the catalytic core domains are displayed, color coded according to structural similarity. Viewed from the major groove side of the acceptor stem, a phosphate backbone outline of the tRNAs is shown (tan), and tRNAPyl is shown in purple. In space filling representation, a glutamyl-adenylate (a) and a pyrrolysyl-adenylate (b) highlight the class I and class II active site pockets, respectively. The small substrates are partially obscured by the protein backbone due to the need to show both aaRS families in the same orientation relative to the tRNA.