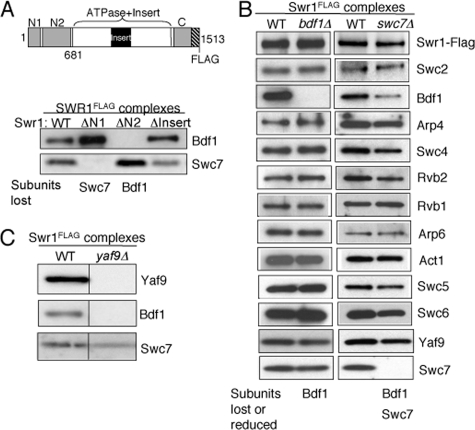
FIGURE 1.
Association of Bdf1 and Swc7 is dependent on the N terminus of Swr1. A, SDS-PAGE and Western blotting analysis of Bdf1 and Swc7 in partial SWR1 complexes purified from wild-type and mutant swr1 strains as indicated. PVDF membrane strips corresponding to subunit(s) were probed with antibodies against Bdf1 and Swc7. The schematic diagram of Swr1 indicates subunit interaction domains of Swr1 and fragments used in this study: N1 (residues 1–210), N2 (residues 278–681), Insert (residues 1002–1221), Swr1 (residues 1–681). SWR1(ΔN1), SWR1(ΔN2), and SWR1(ΔInsert) are mutant SWR1 complexes isolated from cells lacking the N1, N2, and Insert regions of the Swr1 ATPase subunit, respectively (32). B, SDS-PAGE and Western blotting analysis of Swr1, Bdf1, Arp4, Swc4, Yaf9, and Swc7 in SWR1 complexes isolated from wild-type, bdf1Δ and swc7Δ strains. PVDF membranes were probed with anti-Flag (to reveal Swr1), and antibodies against individual SWR1 subunits as indicated. C, SDS-PAGE (4–20% gel) and Western blotting analysis of Yaf9, Bdf1, and Swc7 in SWR1 complexes isolated from wild-type and yaf9Δ cells. PVDF membranes were probed with antibodies against Yaf9, Bdf1, and Swc7, respectively.