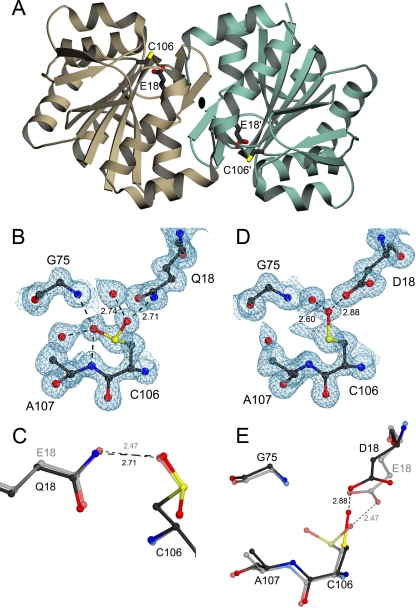
FIGURE 1.
Structural effects of mutations designed to test the hypothesis that
Cys106-sulfinic acid formation is critical to DJ-1 function.
A, a ribbon representation of the DJ-1 dimer, with one
monomer in brown and the other in green. The dimer 2-fold
axis is perpendicular to the page and indicated by an
ellipse. The oxidationprone cysteine (C106) and the
interacting glutamic acid (E18) are represented in each monomer.
B, electron density for the 1.15 Å resolution structure of E18Q
DJ-1 around Cys106 is shown at the 1σ contour level and
calculated with σA weighted coefficients
2mFo - DFc. In E18Q DJ-1,
Cys106 is oxidized to the cysteine-sulfinic acid, where stabilizing
hydrogen bonds are shown as dotted lines with distances given in
Å. C, a superposition of oxidized E18Q (darker model)
and wild-type DJ-1 (lighter model) shows that the key stabilizing
hydrogen bond between residue 18 and
Cys106-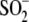 is lengthened in
E18Q DJ-1, weakening this interaction. D, 2mFo -
DFc electron density contoured at 1σ is shown in
blue for the 1.20 Å resolution crystal structure of E18D DJ-1.
Cys106 is oxidized to the easily reduced
Cys106-SO- oxidation product in this variant. In
addition, there is minor electron density that is consistent with either
Cys106-
is lengthened in
E18Q DJ-1, weakening this interaction. D, 2mFo -
DFc electron density contoured at 1σ is shown in
blue for the 1.20 Å resolution crystal structure of E18D DJ-1.
Cys106 is oxidized to the easily reduced
Cys106-SO- oxidation product in this variant. In
addition, there is minor electron density that is consistent with either
Cys106-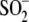 or an alternate
conformation for Cys106-SO-. E, a superposition
of residues in the vicinity of Cys106 in E18D DJ-1 (darker
model) and the corresponding region in oxidized wild-type DJ-1
(lighter model). The E18D substitution results in structural
perturbations at Cys106 that stabilize the
Cys106-SO- oxidation product and hinder further
oxidation. All figures were created using POVscript+
(40).
or an alternate
conformation for Cys106-SO-. E, a superposition
of residues in the vicinity of Cys106 in E18D DJ-1 (darker
model) and the corresponding region in oxidized wild-type DJ-1
(lighter model). The E18D substitution results in structural
perturbations at Cys106 that stabilize the
Cys106-SO- oxidation product and hinder further
oxidation. All figures were created using POVscript+
(40).