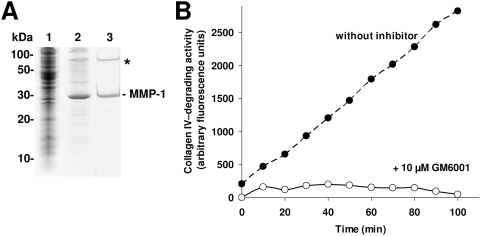
Figure 12. Purification and refolding of recombinant Tribolium MMP-1 and determination of its collagenolytic activity.
(A) MMP-1 (lane 2) was purified to apparent homogeneity from the urea-soluble E. coli inclusion bodies fraction (lane 1) as observed by SDS–PAGE analysis. After refolding in appropriate buffer, we observed the MMP-1 protein band (according to the calculated molecular mass of about 30.3 kDa) and an additional band with an estimated two-fold molecular weight that may correspond to a dimer of MMP-1 (indicated by an asterisk). Molecular mass standards are indicated in kDa. (B) Collagen-IV degrading activity of recombinant Tribolium MMP-1 was monitored using DQ™ collagen (type IV from human placenta). The reaction was found to be linear for at least 100 min under examined conditions. This activity was abolished in the presence of 10 µM GM6001.