Figure 4.
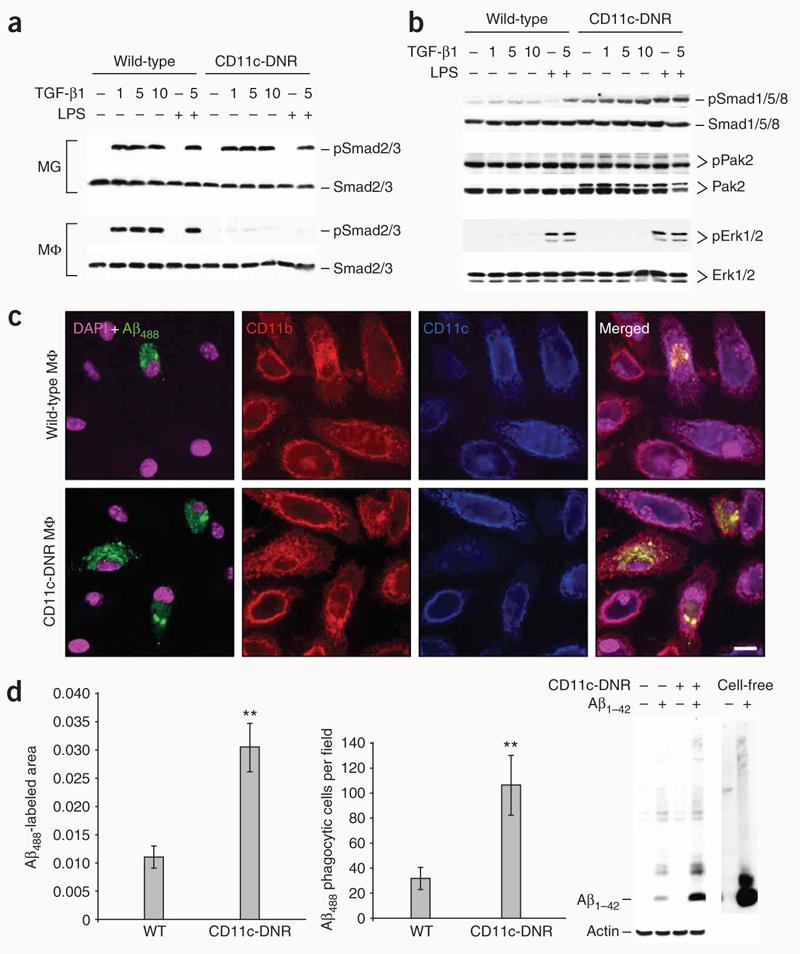
TGF-b1 shifts CD11c-DNR macrophages from canonical to alternate Smad signaling and increases Aβ phagocytosis in vitro. (a) Primary microglia (MG) or macrophages (MΦ) from wild-type or CD11c-DNR mice went untreated or were treated for 30 min with a dose range of recombinant TGF-β1 (1, 5 or 10 ng/ml as indicated) with or without 50 ng/ml of LPS. Cell lysates were western blotted for phosphorylated (p) and total Smad2/3 proteins as an indicator of canonical TGF-β-activated signaling. (b) Primary macrophages were treated as above and western blotted for phosphorylated and total Smad1/5/8 or p21-activated kinase 2 (Pak2; both activated in the alternate Smad signaling pathway), or extracellular signal-regulated kinase (Erk) 1/2. (c) Primary macrophages were pulsed for 4 h with 2 μg/ml of preaggregated Aβ488 and chased for 15 min before analysis by confocal microscopy with antibodies to CD11b or CD11c (merged images are shown on the right). (d) Quantification of confocal images (n = 3 randomly-selected fields per group) was performed, and Aβ488− labeled area is shown on the left. Numbers of Aβ488 phagocytic cells per field are shown in the middle graph. Data are represented as group means ± s.d. Cell lysates were prepared from macrophages treated in parallel with 2 μg/ml of unlabeled human synthetic Aβ1–42, and 2 ng of the peptide (cell-free) was western blotted side-by-side with antibody 6E10 (right). Data shown in (a–d) are representative of three to four independent experiments in which similar results were obtained.
