Abstract
Staphylococcus aureussecretes various toxins that act as superantigens by stimulating a large fraction of the host’s T cells. Toxin binding to variable domains of T cell receptor β chains (Vβ) leads to massive release of inflammatory molecules and potentially to toxic shock syndrome (TSS). Previously, we generated soluble forms of different Vβ domains with a high affinity for binding superantigens. However, a broader spectrum antagonist is required for the neutralization of multiple toxins. In the present study, we expressed Vβ domains in tandem as a single-chain protein and neutralized the clinically important superantigens staphylococcal enterotoxin B and TSS toxin–1 with a single agent.
Staphylococcus aureus and Streptococcus pyogenes secrete a family of structurally-related toxins that bind to the T cell receptor variable region of the β chain (Vβ), and to a major histocompatibility complex (MHC) class II molecule. Toxicity is the result of stimulation of a large fraction of the T cell repertoire and the subsequent release of massive levels of inflammatory cytokines that can lead to toxic shock syndrome (TSS) and organ failure. To date, 19 different superantigens secreted by S. aureus have been identified, each encoded by mobile genetic elements. Two of the most commonly expressed superantigens, each of which has been associated with significant mortality, are staphylococcal enterotoxin B (SEB) and toxic shock syndrome (TSS) toxin–1 (TSST-1) [1].
To develop potential treatments for S. aureus toxin–mediated diseases, studies have explored whether toxoids could elicit antibodies that are capable of neutralizing multiple members of the toxin family [2, 3]. In addition, there have been some studies to generate mouse monoclonal antibodies that cross-react with more than 1 exotoxin [4]. Despite some limited success, it remains difficult to generate a broad-spectrum neutralizing approach because of the structural diversity of these toxins [1]. For example, SEB and TSST-1 share only 22.3% amino acid–sequence identity and they bind to distinctly different Vβ regions of the human T cell receptor (TCR) repertoire [5].
To develop potential neutralizing agents against individual exotoxins, we elected to use single Vβ domains as a platform for engineering picomolar affinity toxin-binding agents. Recently, we have shown that these 12–15-kDa proteins could be generated against both TSST-1 and SEB [6, 7]. Soluble forms of the SEB-reactive Vβ proteins were effective in rabbit models of SEB-induced disease [7]. Because of their small size (less than one-tenth the size of an IgG molecule) and their modular nature, we reasoned that it might be possible to clone different Vβ region domains in tandem to generate a single protein capable of neutralizing multiple toxins. Here we show that a 30-kDa fusion protein of 2Vβ domains, expressed in high quantities from Escherichia coli, was capable of fully neutralizing the in vitro activity of SEB and TSST-1. These proteins thus serve as a potential drug for use in treating S. aureus infections that involve multiple toxins.
Material and methods
SEB, TSST-1, and their biotinylated forms were obtained from Toxin Technology. Monoclonal antibodies against human IL-2 and mouse IL-2 were obtained from BD Biosciences Pharmingen.
G5-8, a mouse Vβ(Vβ8.2) engineered to bind to SEB with an affinity (KD) of 48 pM[7], was polymerase chain reaction (PCR) amplified and cloned into the NcoI and EcoRI sites of the bacterial expression vector pET28a as a His-thrombin-G5-8 fragment. A (Gly4Ser)4linker linker followed by the D10 gene, a human Vβ (Vβ2.1) engineered to bind to TSST-1 with an affinity (K KD) of 180 pM [6], was subcloned at the C-terminus of G5-8 after PCR amplification and cloning into the EcoRI and XhoI sites. The hybrid and individual Vβ proteins were expressed as inclusion bodies from the pET28a expression vector in E. coli BL21(DE3), as described elsewhere [6, 7] (and in the appendix, which is available only in the electronic version).
The binding parameters of the individual Vβ proteins and the fusion protein were determined by surface plasmon resonance analysis (SPR), with immobilized superantigens, as described elsewhere for the individual Vβ proteins [6, 7] (and in the appendix, which is available only in the electronic version).
To measure TSST-1 activity, we used the Jurkat T cell line JRT3–2.1 transfected with the human Vβ2.1 gene, as described elsewhere [8]. JRT3-2.1 cells (10 106 cells/well) were stimulated with various concentrations of TSST-1 in the presence of MHC class II–positive B cell line LG-2 cells (2 × 105 cells/well). Soluble, high-affinity Vβ domains D10 or the G5-8/D10 fusion protein were added at various concentrations. After 18 h, plates were centrifuged, supernatant was collected, and interleukin (IL)–2 levels were measured by ELISA. To measure SEB activity, the mouse T cell hybridoma 58 −/− cells, transfected with the mouse mouse Vβ8.2 gene (the 2C TCR mutant called m6, cotransfected with CD8 αβ genes) (2 × 106 cells per well) [9], LG-2 cells (2 × 105 cells/well), and 50 nmol/L SEB were used in the presence or absence of purified Vβ proteins. After 26 h, plates were centrifuged, supernatants were collected, and IL-2 levels were measured by ELISA. Additional details are provided in the appendix, which is available only in the electronic version.
To evaluate the ability of Vβ proteins to inhibit the activation of primary human T cells by TSST-1 or SEB, both IL-2 secretion and proliferation assays were performed. In the IL-2 assays, inhibitors were added to fresh, gradient-purified human peripheral blood mononuclear cells (PBMCs) stimulated in 24-well plates (5 × 105 cells/well) in the presence of TSST-1, SEB, or both superantigens (each at 25 nmol/L). After 18 h, IL-2 production was measured by ELISA. In the proliferation assays, toxins and/or Vβ proteins were added to PBMCs (2 × 105 cells/well) in 96-well plates, and after 3 days 3H-thymidine was added for 24 h to measure proliferation.
Results
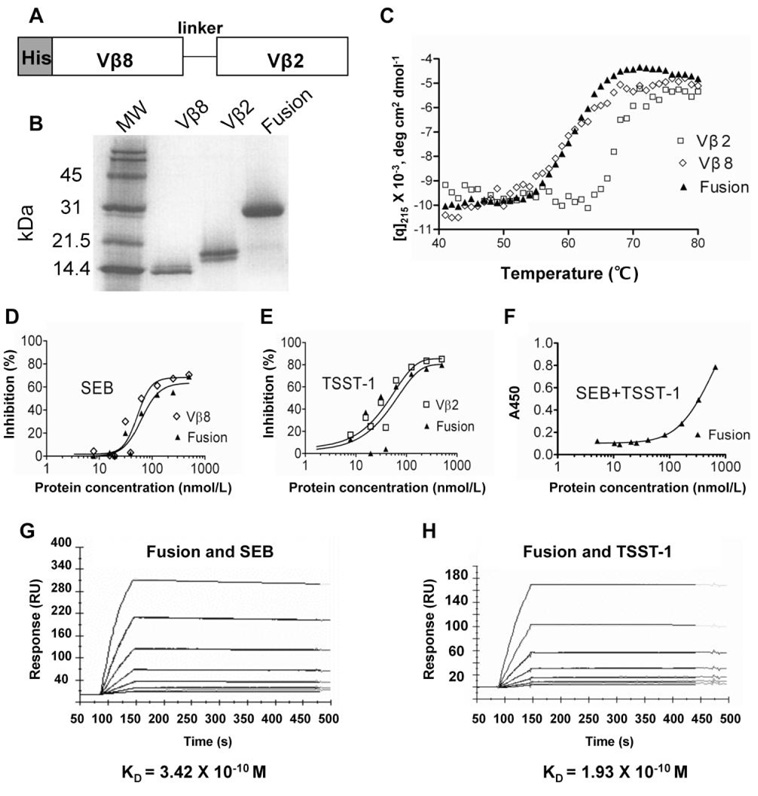
Although it would be highly desirable to neutralize both of the S. aureus exotoxins—TSST-1 and SEB—with 1 agent, they exhibit only 22.3% amino acid–sequence identity and thus there have been no studies that have shown that the toxoids of one can induce neutralization of the other. Furthermore, each toxin is known to exert its toxic effects by binding to completely different Vβ region subfamily members [5]. TSST-1 binds exclusively to human Vβ2+ T cells [10], whereas SEB binds to various human Vβ regions, as well as mouse Vβ8 [11]. To produce soluble neutralizing agents, we have recently engineered these 2 Vβ domains to bind their respective toxins with picomolar affinities [6, 7]. To explore whether we could design a single agent that neutralized both TSST-1 and SEB, we cloned these 2 Vβ domain genes as a single-chain gene with a (Gly4Ser)4 linker (figure 1A), expressed in E. coli and refolded from inclusion bodies. The fusion protein and each of the individual Vβ region proteins were purified by nickel affinity chromatography and gel filtration chromatography. The proteins migrated with the expected monomeric sizes under nondenaturing conditions (data not shown) and by SDS-PAGE (figure 1B). Accordingly, the Vβ proteins migrated at approximately 14–15 kDa and the fusion protein migrated at approximately 30 kDa. Thermal denaturation experiments showed that all 3 of the proteins exhibited excellent thermal stability, with TM values (temperature at one-half maximal denaturation) of 61°C (G5-8 and fusion protein) and 68°C (D10) (figure 1C).
Figure 1.
Purification and binding characterization of a multidomain Vβ fusion protein specific for staphylococcal enterotoxin B (SEB) and toxic shock syndrome toxin–1 (TSST-1). A, Diagram of the single-chain construct. B, SDS-PAGE gel of individual Vβ domains and the fusion protein. C, Thermal stabilities of the individual Vβ proteins and the fusion protein determined using circular dichroism. D, Competition ELISA of SEB binding. E, Competition ELISA of TSST-1 binding. F, Direct binding ELISA with SEB absorbed to wells, followed by the fusion protein, followed by biotinylated TSST-1. G, Surface plasmon resonance (SPR) analysis of the Vβ fusion–SEB interaction. H, SPR analysis of the Vβ fusion–TSST-1 interaction. Details of the procedures are described in the appendix, which is available only in the electronic version. RU, resonance units.
To determine whether the fusion protein contained properly folded Vβ domains, we performed competition ELISAs with immobilized individual domains and biotinylated SEB or TSST-1 (figure 1D and 1E). The fusion protein bound to SEB and TSST-1 with an effectiveness similar to that of the single Vβ domains. In addition, the fusion protein could bind simultaneously to TSST-1- and SEB, as determined in an ELISA with immobilized SEB and biotinylated TSST-1 as detecting agent (figure 1F).
To measure the binding affinities of the 2 Vβ domains in the fusion protein, we performed SPR analysis with immobilized TSST-1 and SEB (figure 1G and 1H). The results showed that both Vβ domains in the fusion protein bound to their respective ligands with picomolar affinities. The fusion protein exhibited a KD value for TSST-1 of ~190 pM, similar to that measured for the individual Vβ2.1 D10 domain (KD, 180 pM). The fusion protein exhibited a KD value for SEB of ~340 pM, approximately 7-fold lower than the affinity of the individual Vβ8 G5-8 domain (KD, 50 pM). It is unclear what caused this modest reduction in affinity of the Vβ8 domain, but it is possible that Vβ2-Vβ8 interactions might influence the affinity of the domains and that shortening the length of the linker would minimize this effect (similar to the strategy used for single-chain variable fragment “diabodies” to prevent intrachain VH–V L associations) [12].
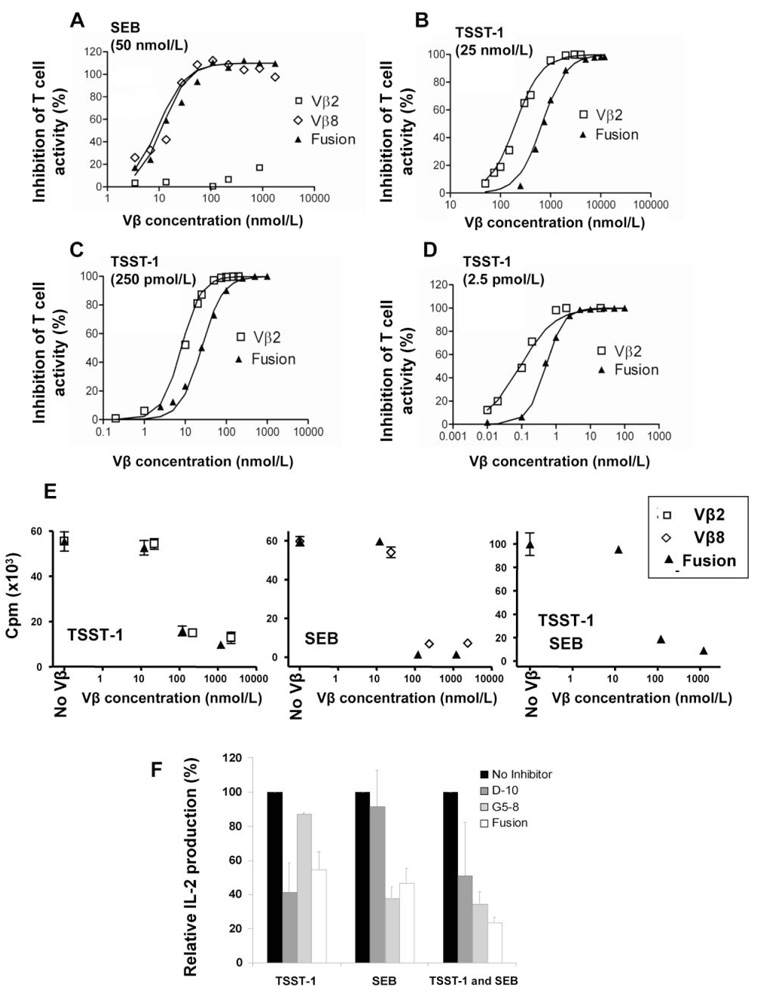
Because the fusion protein exhibited SEB and TSST-1 binding activity, we performed various in vitro T cell assays to determine whether the single-chain protein could neutralize both SEB and TSST-1. In the first assays, 2 transfected T cell lines, expressing either the mouse Vβ8.2 TCR, or the human Vβ2.1 TCR, were used to assay activity mediated by the toxins SEB and TSST-1, respectively. In the presence of the human class II+ antigen-presenting LG-2 cells and various concentrations of either SEB or TSST-1, both cell lines secreted IL-2, which was measured by a capture ELISA. SEB was assayed at a concentration of 50 nmol/L, in the presence of various concentrations of the 3 Vβ proteins, Vβ8.2 G5-8, Vβ2.1 D10, or the fusion protein. Both G5-8 and the fusion protein completely inhibited the activity of SEB, with nearly equal effectiveness (figure 2A). As expected, the TSST-1–reactive Vβ D10 was unable to inhibit SEB-mediated activity at any concentration. A similar experiment was performed with TSST-1 and the Vβ2.1 T cells, except that the concentrations of TSST-1 used were analyzed over a broader range (2.5 pmol/L to 25 nmol/L) due to the potency of this toxin in the in vitro T cell assay (figure 2B–2D). Even at the highest concentration of TSST-1 (25 nmol/L), both the soluble Vβ2.1 D10 domain and the fusion protein were capable of complete inhibition. As expected, higher concentrations of Vβ proteins were required for complete neutralization at 250 pmol/L and 25 nmol/L TSST-1, because the toxin is active even under conditions in which there are very low levels of unbound TSST-1 (e.g., at a concentration of 25 nmol/L, even 0.01% free toxin would be capable of stimulating the T cells). The high affinity of the Vβ2.1 D10 domain no doubt accounts for such potency over a range of TSST-1 concentrations.
Figure 2.
Functional neutralization of T cell activation by staphylococcal enterotoxin B (SEB) and toxic shock syndrome toxin–1 (TSST-1). A, Mouse Vβ8.2 + T cells and SEB at 50 nmol/L. B, Human Vβ2.1 + T cells and TSST-1 at 25 nmol/L. C, Human Vβ2.1 + T cells and TSST-1 at 250 pmol/L. D, Human Vβ2.1 + T cells and TSST-1 at 2.5 pmol/L. Soluble, high-affinity Vβ2 D10 (open square), Vβ8 G5-8 (open diamond), or the fusion protein G5-8/D10 (solid triangle) were added at the indicated concentrations to wells that contained the toxin, T cells, and antigen presenting cells, as described in Materials and Methods. Interleukin (IL)-2 released after 18 h (TSST-1 assay) or 26 h (SEB assay) was measured by ELISA and percentage inhibition in the presence of the Vβ preparation was calculated as follows: {[(A450–A570)no inhibitor −(A450–A570)inhibitor]/[A450–A570]no inhibitor} × 100. For human peripheral blood mononuclear cell assays, proliferation (E) or IL-2 secretion (F) was measured as described in Materials and Methods. T cell proliferation was assessed with SEB or TSST-1 (80 nmol/L) in the presence or absence of various Vβ protein concentrations. IL-2 production was assessed with SEB or TSST-1 (25 nmol/L) in the presence or absence of Vβ proteins (1 µmol/L). Vβ proteins alone (without toxin) did not induce IL-2 or proliferation above background (not shown). Data are expressed as the mean (± SE values) of 3 independent experiments (IL-2) or from 4 replicate samples (proliferation).
In the other in vitro assay, human PBMCs were stimulated with SEB, TSST-1, or both SEB and TSST-1, and the Vβ proteins were examined for their ability to inhibit T cell proliferation (figure 2E) or IL-2 secretion (figure 2F). In each assay, the individual Vβ domains against the specific toxin and the fusion protein were capable of inhibiting the polyclonal T cell activity stimulated by the toxins. The neutralization of toxin activity occurred at low Vβ concentrations, just above the stoichiometric level of toxin (e.g., 130 nmol/L of fusion protein and 80 nmol/L toxin) (figure 2E).
Discussion
The neutralization of different allelic forms of the same toxin or multiple different toxins secreted by one or more organisms can represent a major problem for vaccination or treatment regimens. Despite S. aureus’s potential for the secretion of numerous toxins, there is some evidence that SEB and TSST-1 are among the most clinically damaging toxins [13]. Because of their differences with respect to amino acids and serotypes, it has been difficult to develop a single toxoid or monoclonal antibody that would be effective against both toxins. To develop effective neutralizing agents, we have recently shown that soluble forms of the Vβ receptors can be engineered to picomolar affinity against TSST-1 [6] and SEB [7]; one of the Vβ mutants (G5-8) was shown to be effective in various rabbit models of SEB-induced TSS [7].
Although we have shown in the present study that a protein consisting of 2 tandem Vβ regions was a potent antagonist of SEB and TSST-1 in vitro, it would be possible to generate fusion proteins with additional neutralizing domains so that more than 2 toxins could be neutralized. It remains to be seen whether the increase in size above a single Vβ region results in reduced in vivo efficacy, but it would be possible to engineer linker regions that could be cleaved in vivo if this were the case. One significant advantage that such fusions have over the use of separately expressed and purified Vβ regions is the ease of developing a single product in an E. coli expression system. The use of intravenous immunoglobulin as an adjunct therapy for exotoxin neutralization suffers from both the amounts of Ig required and the expense. Recent developments with analogous V region heavy-chain domains have highlighted the value of easily expressed small proteins as alternatives to full-length IgG molecules [14, 15].
Acknowledgments
Financial support: National Institutes of Health (grants AI064611 to D.M.K. and AI55882 to E.J.S.; training grant T32 GM07283 to R.A.B.); Canadian Institute of Health Research (grant MOP-64176 and New Investigator award to J.K.M); Boston Biomedical Research Institute (Scholar Award to B.M.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Boles JW, Pitt ML, LeClaire RD, et al. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin Immunol. 2003;108:51–59. doi: 10.1016/s1521-6616(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 3.Burnett JC, Henchal EA, Schmaljohn AL, Bavari S. The evolving field of biodefence: therapeutic developments and diagnostics. Nat Rev Drug Discov. 2005;4:281–297. doi: 10.1038/nrd1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang LT, Kum WW, Chow AW. Inhibition of staphylococcal enterotoxin B-induced lymphocyte proliferation and tumor necrosis factor α secretion by MAb5, an anti-toxic shock syndrome toxin 1 monoclonal antibody. Infect Immun. 2000;68:3261–3268. doi: 10.1128/iai.68.6.3261-3268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischer B, Necker A, Leget C, Malissen B, Romagne F. Reactivity of mouse T-cell hybridomas expressing human Vβ gene segments with staphylococcal and streptococcal superantigens. Infect Immun. 1996;64:987–994. doi: 10.1128/iai.64.3.987-994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonpane RA, Moza B, Sundberg EJ, Kranz DM. Characterization of T cell receptors engineered for high affinity against toxic shock syndrome toxin-1. J Mol Biol. 2005;353:308–321. doi: 10.1016/j.jmb.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Buonpane RA, Churchill HR, Moza B, et al. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat Med. 2007;13:725–729. doi: 10.1038/nm1584. [DOI] [PubMed] [Google Scholar]
- 8.Rahman AK, Herfst CA, Moza B, et al. Molecular basis of TCR selectivity, cross-reactivity, and allelic discrimination by a bacterial superantigen: integrative functional and energetic mapping of the SpeC-V β2.1 molecular interface. J Immunol. 2006;177:8595–8603. doi: 10.4049/jimmunol.177.12.8595. [DOI] [PubMed] [Google Scholar]
- 9.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 10.Choi Y, Lafferty JA, Clements JR, et al. Selective expansion of T cells expressing Vβ2 in toxic shock syndrome. J Exp Med. 1990;172:981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrack P, Kappler J. The staphylococcal entertoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 12.Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–64448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 14.Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM. Domain antibodies: proteins for therapy. Trends Biotechnol. 2003;21:484–490. doi: 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]