Abstract
Obesity is currently a worldwide epidemic and public health burden that increases the risk for developing insulin resistance and several chronic diseases such as diabetes, cardiovascular diseases and non-alcoholic fatty liver disease. The multifactorial causes of obesity include several genetic, dietary and lifestyle variables that together result in an imbalance between energy intake and energy expenditure. Dietary approaches to limit fat intake are commonly prescribed to achieve the hypocaloric conditions necessary for weight loss. But dietary fat restriction is often accompanied by increased carbohydrate intake, which can dramatically increase endogenous fatty acid synthesis depending upon carbohydrate composition. Since both dietary and endogenously synthesized fatty acids contribute to the whole-body fatty acid pool, obesity can therefore result from excessive fat or carbohydrate consumption. Stearoyl-Coenzyme A desaturase-1 (SCD1) is a delta-9 fatty acid desaturase that converts saturated fatty acids into monounsaturated fatty acids (MUFA) and this activity is elevated by dietary carbohydrate. Mice lacking Scd1 are protected from obesity and insulin resistance and are characterized by decreased fatty acid synthesis and increased fatty acid oxidation. In this review, we address the association of high-carbohydrate diets with increased SCD activity and summarize the current literature on the subject of SCD1 and body weight regulation.
Keywords: Stearoyl-CoA Desaturase, SCD1, Obesity, Insulin, Carbohydrate, Lipogenesis
1. Introduction
Obesity is currently a worldwide epidemic prevalent in both adults and children that is caused by an imbalance of high energy consumption with low energy expenditure [1]. Increased weight gain is associated with an insulin resistance syndrome that elevates the risk for several chronic diseases including diabetes, cardiovascular diseases and non-alcoholic fatty liver disease [2, 3]. In response to positive energy balance, the adipose tissue stores this surplus energy primarily in triglyceride-rich lipid droplets. Sustained nutrient overload is hypothesized to impair normal adipocyte function leading to abnormal adipokine production and leakage of nutrients from the adipocyte into insulin-sensitive tissues such as muscle and liver [4]. These events contribute to both local and systemic insulin resistance resulting in hyperinsulinemia, increased hepatic glucose production, reduced peripheral glucose uptake and dyslipidemia [2, 5, 6].
Alterations in dietary macronutrient composition and caloric intake can be effective treatments for weight loss and plasma lipid management. Most food-based recommendations disseminated to the general public share a consistent message to consume at least 55% of calories from carbohydrate, increase consumption of fruits, vegetables, legumes and whole grains, but minimize intake of sugar, cholesterol, saturated and trans fatty acids [7, 8]. Many popular weight-loss strategies share some of these recommendations, but their target dietary carbohydrate intakes span a wide range between 22.2% and 81.0% [9]. While dietary adherence is an important factor in the efficacy of any intervention, individual variation in response to dietary changes also influences the outcome [10]. For example, interactive effects between genetic modifiers and low-fat, high-carbohydrate diets can effect levels of plasma very low-density lipoprotein (VLDL) triglyceride levels, low-density lipoprotein (LDL) subclass distribution and insulin secretion response [11–13].
One of the gene-nutrient interactions that may be responsible for the differential response to nutritional intervention is the dietary modulation of stearoyl-Coenzyme A desaturase (SCD). SCD is a delta-9 fatty acid desaturase that converts saturated fatty acids into monounsaturated fatty acids (MUFA) [14]. These MUFA are key substrates for the formation of complex lipids such as triglycerides, cholesterol esters, wax esters and diacylglycerols. Additionally, increased cellular SCD1 activity has been suggested to influence fatty acid partitioning by promoting fatty acid synthesis but decreasing oxidation [15–17]. Previous studies have shown that the reduced MUFA synthesis that occurs in Scd1-deficient mice or those treated with Scd1-targed antisense oligonucleotides is associated with several metabolic changes that elicit protection from obesity, cellular lipid accumulation and insulin resistance [14, 17–21]. Scd1 levels are elevated by both dietary and hormonal factors, such as glucose, fructose, saturated fatty acids and insulin, but repressed by polyunsaturated fatty acids (PUFA) and leptin [14, 16, 22–25]. Therefore, high levels of Scd1 elicited by both dietary carbohydrate and certain fatty acids may be a significant contributor to the development of obesity and other chronic diseases.
This review addresses the association of high-carbohydrate diets with obesity and increased SCD activity. The first part of this review clarifies several nutritional variables that comprise a high-carbohydrate diet that can impact its metabolic effect. In particular, we emphasize the distinction between low- and high-glycemic index carbohydrate sources in relation to obesity. Next, we discuss how these nutritional components and the associated hormonal changes regulate the expression of SCD1. The final part of this review will summarize the current literature on the subject of SCD1 and body weight regulation.
2. Defining the high-carbohydrate diet
2.1 The origins of the low-fat, high-carbohydrate diet
The recommendation to substitute carbohydrate calories for fat calories is partly rooted in studies from both human and animal models that showed a plasma LDL-cholesterol raising effect of the dietary saturated fats lauric (12:0), myristic (14:0), and palmitic acid (16:0) compared to unsaturated fats [26–28]. One strategy to limit saturated fat intake involves the isocaloric substitution of dietary unsaturated fat for saturated fat. The low-fat, high-carbohydrate diet is an extension of this approach that limits total fat intake as opposed to targeting saturated fat. This is achieved by exchanging dietary fat with carbohydrate or protein. Since fat is more calorie dense (~9 kcal/g) than carbohydrate or protein (~4 kcal/g), this can be a useful strategy to reduce caloric intake to promote negative energy balance and weight loss. For the purpose of this review, changes in carbohydrate and fat calories are assumed to occur in the absence of altered protein intake. Thus, the low-fat, high-carbohydrate approach results in a substantial increase in the percent calories coming from carbohydrate at the expense of fat. However, it is important to note that increased dietary protein intake at the expense of carbohydrate or fat calories has been associated with increased satiety and compliance with weight loss and weight maintenance strategies [29, 30].
2.2 Not all carbohydrates are created equal
The metabolic response to carbohydrate intake is influenced by both carbohydrate mass as well as carbohydrate composition [31, 32]. Carbohydrates exist as monosaccharides (i.e. glucose, fructose and galactose), disaccharides (i.e. sucrose and lactose), or longer polymers of glucose (i.e. maltodextrins and starches). Dietary monosaccharides are readily absorbed by enterocytes in the upper small intestine and rapidly transported out the cell into the portal circulation. Disaccharides must first be cleaved into monosaccharides by brush-border glucosidases prior to absorption. For example, dietary sucrose is cleaved by sucrase to yield equal amounts of glucose and fructose. High-fructose corn syrup is a processed sugar derived from corn starch that contains approximately equal amounts of glucose and fructose. Increased consumption of simple sugars such as sucrose and high-fructose corn syrup that rapidly enter the bloodstream after ingestion has been suggested to be a large contributor to the increased incidence of obesity and the metabolic syndrome [33].
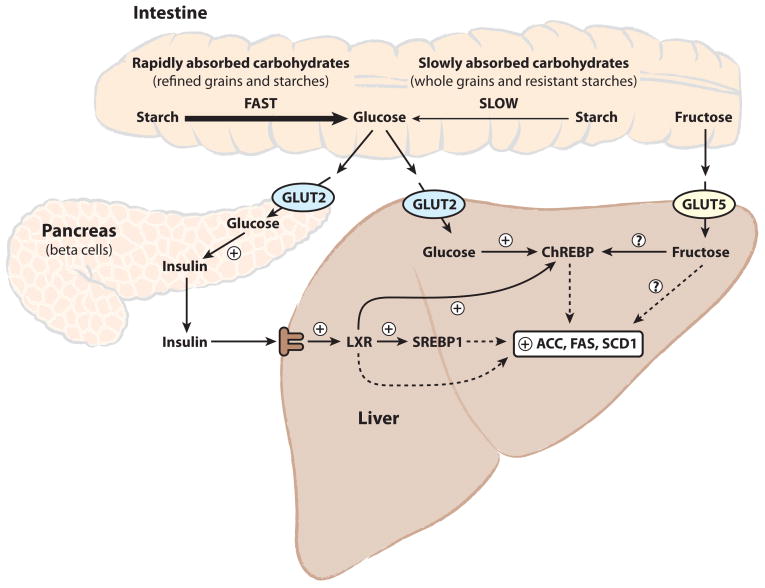
In contrast, plant starches are complex carbohydrates consisting of hundreds to several thousands of glucose units linked by α-1,4-glycosidic bonds (amylose), or a branched form linked by both α-1,4- and α-1,6-glycosidic bonds (amylopectin). These starches require salivary-and pancreatic-derived amylase enzymes to cleave the glycosidic bonds and convert starch into maltose, maltotriose, and α-dextrins (or maltodextrins), which are further hydrolyzed to glucose by brush border enzymes. The physical accessibility of the carbohydrate in foods to hydrolytic enzymes is an important factor in controlling the rate of monosaccharide entry into the bloodstream. The amylose:amylopectin ratio of starch can influence carbohydrate absorption due to the ability of amylose-rich starches to form more tightly packed, hydrolysis-resistant granules [34]. The physical form (liquid vs. solid), degree of processing (whole vs. refined), method of preparation (raw vs. cooked) and fiber content can also have a significant impact on the rate of carbohydrate absorption [34]. These variables are often expressed as the glycemic index of a food, which is defined as the blood glucose response elicited by a test food relative to a glucose standard containing an equivalent mass of carbohydrate [35]. Both the glycemic index as well as the total carbohydrate content of a food will influence postprandial glycemia and elicit a proportional insulin secretory response (Figure 1) [32].
Figure 1.
High-carbohydrate diets induce hepatic lipogenesis via direct and indirect liver nutrient sensing mechanisms. After carbohydrate ingestion, starches are broken down in the intestine into glucose and transported into the bloodstream. The blood glucose load is influenced by both the total carbohydrate intake as well as the rate of starch digestion. Simple sugars such as glucose, fructose, and sucrose require minimal digestion, unlike starches, and are rapidly absorbed. Glucose-sensing by the pancreatic β-cells results in a proportional increase in insulin secretion. Hepatic sensing of glucose and fructose together with increased hepatic insulin-signaling promotes the activation of ChREBP, LXR, and SREBP to induce hepatic lipogenic gene expression, including SCD1.
3. Nutritional control of lipogenesis and the role of SCD1
3.1 Cellular sensing and fate of dietary carbohydrate
The transport of glucose into the cell and subsequent phosphorylation to glucose-6-phosphate are the primary rate-limiting steps for glucose metabolism. Pancreatic β-cells and liver hepatocytes, as well as some cells of the hypothalamus, kidney, and small intestine, express the high Km plasma membrane GLUT2 glucose transporter and glucokinase glucose phosphorylating enzyme. This allows these cells to act as “glucose-sensors” by coupling millimolar changes in blood glucose concentrations to corresponding signal-generating metabolic flux rates. For example, dietary modifications resulting in an increased blood glucose load elicit a greater β-cell insulin secretory response [32, 36] (Figure 1). It is important to note that amino acids and fatty acids are relatively weak insulin secretagogues alone, but can also acutely augment glucose-stimulated insulin secretion [37–40]. In contrast to β-cells and hepatocytes, tissues such as muscle and adipose require insulin to stimulate glucose uptake by promoting the translocation of a large pool of intracellular GLUT4 glucose transporters to the cell surface for glucose transport and subsequent phosphorylation by the low Km hexokinase [41]. One fate of glucose-6-phosphate is to be utilized to replenish cellular glycogen stores in tissues such as muscle and liver. However, adipose tissue and liver also have a large capacity for lipogenesis and an alternative fate of glucose-6-phosphate is the conversion to acetyl-CoA for fatty acid synthesis and storage as triglycerides.
3.2 Stimulation of hepatic de novo lipogenesis by dietary carbohydrate
While both adipose and liver may perform de novo lipogenesis (DNL), the liver is unique in its ability to dramatically increase fatty acid synthesis in response to changes in dietary macronutrient intake. Of particular interest is the effect of dietary carbohydrate, especially sugars, in humans to increase hepatic DNL [42]. Hepatic DNL is lowest after fasting and peaks in the postprandial period [43], but is also strongly influenced by dietary composition. Whereas minimal DNL occurred on a high-fat diet (40% of calories as fat, 45% as glucose polymers), hepatic fatty acid synthesis was markedly increased upon low-fat feeding (10% of calories as fat and 75% as glucose polymers) [44]. Furthermore, eucaloric substitution of dietary starch for sugar reduced hepatic DNL [45]. Additionally, fructose has been shown to be a more potent inducer of hepatic lipogenesis than glucose in mice, rats and humans [24, 46–48]. This is partly due to the remarkable ability of the liver to clear approximately 70% of blood fructose [49]. Also, fructose metabolism is not inhibited at the level of phosphofructokinase as is glucose, allowing for fructose to bypass this regulator step and serve as an unregulated source of precursors for hepatic lipogenesis [33].
The rate of intestinal carbohydrate absorption into the circulation also influences the physiological response to ingested carbohydrate (Figure 1). Behall et al. investigated the effect of slowly absorbed amylose starch compared to rapidly absorbed amylopectin starch in humans and found that consumption of an amylose containing meal elicited a lower postprandial glucose and insulin response compared to those fed an equivalent amount of amylopectin [50–52]. Furthermore, a meta-analysis of 37 prospective human cohort studies found that both high-glycemic index and high-glucose load diets are independently associated with increased risk for several chronic diseases, including type 2 diabetes and coronary heart disease, suggesting that postprandial glycemia and insulin secretion contribute to disease progression [31] This relationship is also exemplified by studies in mice and rats, in which feeding amylopectin starch promoted more adiposity, hepatic steatosis, hyperinsulinemia, and increased plasma triglycerides compared to feeding amylose starch [53–55].
3.3 Transcriptional control of Scd1 by dietary carbohydrate and fatty acids
The amenability of the rodent animal model to both dietary and genetic manipulation has allowed for the elucidation of several molecular mechanisms involved in carbohydrate-induced lipogenesis. One mechanism for elevated hepatic lipogenesis in response to dietary carbohydrate is increased insulin secretion, which promotes the activation of the lipogenic transcription factors liver-X-receptor (LXR) and sterol regulatory element binding protein 1c (SREBP-1c) [56, 57]. LXR activation has also been shown to increase the expression of mRNAs encoding both SREBP-1c and carbohydrate responsive element binding protein (ChREBP) [58]. Increased hepatic glucose flux promotes the formation of xyulose-5-phosphate via the hexose monophosphate shunt to promote activation of protein phosphatase 2A and subsequent dephosphorylation of ChREBP to an active lipogenic transcription factor [48, 59, 60]. Together, SREBP, LXR and ChREBP act to couple increased carbohydrate intake with induction of lipogenic genes including liver pyruvate kinase (L-PK; Pklr), acetyl-CoA carboxylase (ACCα Acaca, ACCβ; Acacb), fatty acid synthase (FAS; Fasn) and Scd1 (Figure 1).
The effect of dietary carbohydrates, such as glucose, fructose and sucrose, to robustly increase hepatic Scd1 is due to both SREBP-1c-dependent and independent mechanisms [21, 24, 61]. Both fructose feeding [24] and LXR agonists [62, 63] have been shown to increase hepatic Scd1 in both SREBP-1c+/+ and SREBP-1c−/− mice. ChREBP deficiency also abrogates the increase in Scd1 elicited by both dietary carbohydrate [64] and LXR agonists [58], although the presence of carbohydrate responsive element in the promoter of Scd1 has yet to be identified. Scd1 expression has been shown to be positively regulated by direct LXR binding to an LXR-response element in the Scd1 promoter, as well as LXR-mediated activation of SREBP-1c transcription [61, 62]. Consistent with fructose being more lipogenic than glucose [46–48], fructose is also a more potent inducer of hepatic Scd1 [24]. As opposed to hepatic carbohydrate metabolism, intracerebroventricular glucose administration strongly decreases hepatic Scd1 [65]. However, the physiological significance of this brain-liver circuit in the context of concomitant direct insulin and glucose effect on the liver remains to be established.
In addition to metabolic effects elicited by dietary carbohydrate, fatty acids also modulate the transcriptional activation of Scd1 and other lipogenic genes [23, 25]. The binding of SREBP-1 to the SREBP response element of the Scd1 promoter is decreased by dietary n-3 and n-6 PUFA, partly due to post-translational repression of SREBP-1c maturation [61]. PUFA also act to decrease mRNA abundance of SREBP-1c potentially by accelerating mRNA decay [66, 67]. Additionally, n-3 and n-6 PUFA decrease the nuclear abundance of ChREBP; however, the mechanism is still unresolved [68, 69]. In contrast to the anti-lipogenic effects of PUFA, dietary saturated fat strongly induces Scd1 expression in a mechanism that may involve fatty acid upregulation of PGC1-βand subsequent coactivation of SREBP-1c and LXR [16, 70]. Thus, the lipogenic potential of a low-fat, high-carbohydrate diet may be increased or decreased by relative amounts of dietary saturated fats and PUFA, respectively.
Recently, Cao et al. have suggested that one product of SCD1, palmitoleate, produced and released by the adipose tissue suppresses hepatic lipogenic rates through a specific inhibition of Scd1 in liver [71]. In their genetic model, mice lacking two major adipocyte fatty acid binding proteins (ap2 and mal1) showed a diminished fatty acid binding capacity in the adipose tissue [72] associated with a 2- to 3-fold increase in palmitoleate content of adipose lipids and plasma free fatty acids and robust repression of hepatic Scd1 [71]. This study also reported reduced hepatic Scd1 in mice after tripalmitoleate infusion compared to tripalmitate infusion [71]. However, the abundant dietary MUFA oleic acid has been previously shown to have no repressive effect on hepatic Scd1 expression relative to the well-documented effects of n-3 and n-6 PUFA linoleic, linolenic, arachidonic, eicosapentaenoic, and docosahexaenoic acid [61, 73–75].Additionally, increased plasma palmitoleate in humans has been independently associated with both hypertriglyceridemia and abdominal adiposity [76]. Therefore, further in vivo validation is necessary to directly evaluate the ability of altered circulating palmitoleate levels to elicit metabolic changes and repress hepatic Scd1 compared to both oleate and n-3 and n-6 PUFA.
4. The relationship of SCD1 to carbohydrate-induced adiposity
4.1 Lessons from Scd1-deficient mice
Mice with a targeted deletion of Scd1 or with spontaneous asebia mutations in Scd1 have provided several novel insights into the metabolic role of SCD1 [19, 77]. Scd1-deficient mice have reduced hepatic fatty acid and triglyceride synthesis in response to high-carbohydrate feeding [18, 21, 24]. This is partly due to decreased maturation of SREBP-1 protein and expression of mRNAs encoding fatty acid synthesis genes [16, 24]. Normal SREBP-1c activation in Scd1−/− mice is restored by feeding high levels of dietary MUFA, but not saturated fat, indicating that induction of the hepatic lipogenic program requires adequate levels of MUFA derived from the diet or from endogenous synthesis [16, 24]. We have recently shown that liver-specific deletion of Scd1 using the Cre-loxP system is also sufficient to block this carbohydrate-induced lipogenic program [78].
The lean phenotype of Scd1-deficient mice also results from increased energy expenditure and oxygen consumption due to enhanced fatty acid oxidation and thermogenesis in liver, muscle, and brown adipose tissue, through both transcriptional and post-transcriptional mechanisms [20, 79–82]. Scd1-deficient mice also have elevated AMP-activated protein kinase (AMPK) activity in muscle and liver, which results in inhibitory phosphorylation of acetyl-CoA carboxylase, reduced malonyl-CoA levels and enhanced import of fatty acids into the mitochondria for oxidation [16, 80, 81, 83]. The mechanism for how cellular MUFA homeostasis regulates the SREBP-1c maturation and AMPK activation are currently unknown.
4.2 Liver-specific loss of Scd1 reveals a role in carbohydrate-induced adiposity
The decreased fatty synthesis and increased fatty acid oxidation phenotypes elicit resistance to obesity and hepatic steatosis in whole-body Scd1-deficient mice when stressed with a high-fat (60% calories from fat) or high-carbohydrate diet (10% calories from fat) [16, 20, 78]. However, liver-specific Scd1-deficient mice are protected from high-carbohydrate, but not high-fat diet-induced obesity and hepatic steatosis [78]. We can conclude from this study that SCD1 activity in extrahepatic tissues is involved in obesity resistance from high-fat diets. Hepatic Scd1 deficiency is apparently insufficient to induce the hypermetabolism phenotype that occurs in whole-body Scd1-deficient mice. Binczek et al. have proposed that disruption of the epidermal lipid barrier in whole-body Scd1-deficient mice is responsible for the obesity resistance [84]. However, metabolic effects derived from inhibition of Scd1 in the adipose or other tissues are also possible. This is supported by the studies of Jiang et al. that reported antisense oligonucleotide-mediated inhibition of Scd1 in the liver and adipose protected mice from high-fat diet-induced obesity without concomitant skin abnormalities [17]. Interestingly, ap2/mal1 knockout mice are protected from high-fat diet induced obesity, insulin resistance, and hepatic triglyceride accumulation, coincident with elevated plasma palmitoleate levels and transcriptional repression of hepatic Scd1 [71]. However, these findings are not in accordance with the lack of high-fat diet-induced obesity resistance observed in liver-specific Scd1-deficient mice [78]. Furthermore, whole-body Scd1-deficient mice have decreased plasma palmitoleate levels despite obesity resistance and reduced hepatic lipogenesis [20, 85]. This suggests that the ap2/mal1 knockout model is causing obesity resistance independent of altered plasma palmitoleate levels and hepatic Scd1 repression.
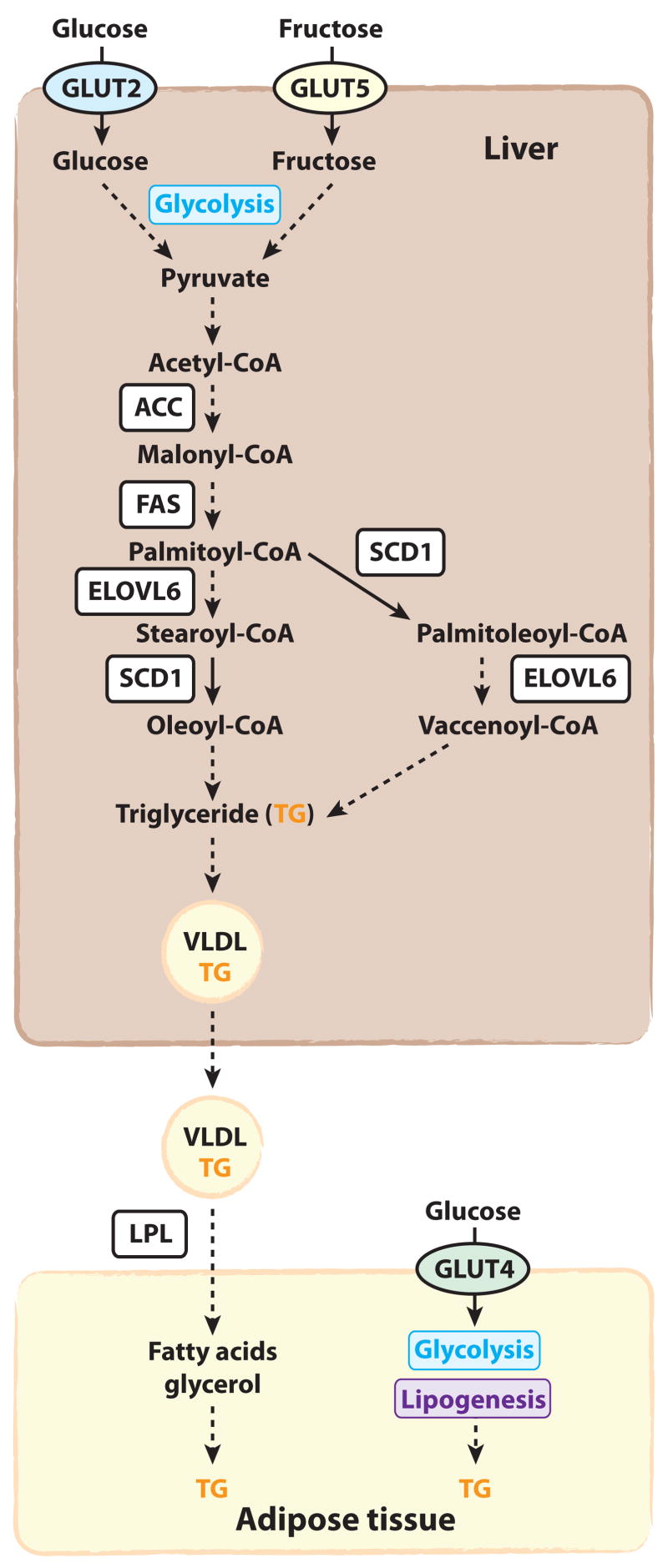
The reduced carbohydrate-induced adiposity in liver-specific Scd1-deficient mice suggests that the hepatic conversion of carbohydrate to fatty acids is requisite for not only hepatic triglyceride synthesis but also expansion of adipose stores (Figure 2). The liver packages its lipid stores into triglyceride-rich VLDL particles, which are secreted into the circulation for hydrolysis by lipoprotein lipase in peripheral tissues [86]. Relative to control mice, liver Scd1-deficient mice fed the high-carbohydrate diet (10% calories from fat) had lower hepatic lipogenic induction, hepatic triglyceride accumulation, and fasting plasma triglycerides [78]. These phenotypes all corresponded with smaller white adipose tissue depots. Similar results were also observed in whole-body Scd1-deficient mice fed a very low-fat, high-carbohydrate diet. This is reminiscent of studies by Scribner et al. in which long-term feeding of a high-glycemic index diet to mice promoted higher hepatic triglycerides, plasma triglycerides and adiposity relative to mice fed a low-glycemic index diet, presumably due to differential induction of hepatic lipogenesis [54, 55]. This data suggests that the transport of de novo synthesized hepatic triglycerides to the adipose tissue must occur in order for high-carbohydrate diets to promote weight gain (Figure 2). However, a dysregulation of hepatic carbohydrate metabolism or insulin signaling may also contribute to decreased adiposity observed in these models.
Figure 2.
In response to increased carbohydrates and insulin, the hepatic levels of lipogenic enzymes are increased (Figure 1). The augmented lipogenic capacity of the liver allows for the large load of dietary carbohydrate to be converted to fatty acids for storage as triglyceride (TG) and secretion as VLDL. SCD1 catalyzes the conversion of the saturated fatty acid products of FAS into MUFA, which are the preferred substrate for triglyceride synthesis. Peripheral tissues such as adipose tissue take up these triglycerides from VLDL in a lipoprotein-lipase (LPL) dependent mechanism. Adipocytes can also uptake glucose in a GLUT4-dependent mechanism for conversion to triglycerides.
4.3 Metabolic effects of a very-low fat, high-carbohydrate diet in mice
Most rodent standard or purified low-fat, high-carbohydrate diets contain at least 10% of calories from fat and supply sufficient MUFA and PUFA for survival. We have recently explored the metabolic effect of feeding a high-sucrose, very low-fat (HSVLF) diet for 2 weeks in whole-body and liver-specific Scd1-deficient mice [78, 87, 88]. This diet contributes ~2.5% calories from fat (derived from corn oil), which limits total fat as well as dietary MUFA and PUFA. In response to the HSVLF diet, control mice are able to upregulate de novo lipogenesis in order to synthesize sufficient MUFA to maintain metabolic homeostasis. However, whole-body Scd1-deficient mice develop a complex phenotype involving loss of body weight, hypoglycemia, depleted hepatic glycogen, hypercholesterolemia, and cholestasis [87]. Subsequent hepatic gene expression analysis revealed significant endoplasmic reticulum stress and inflammation that are associated with the metabolic dysfunction [88]. Supplementation of the HSVLF diet with dietary unsaturated fat, but not saturated fat, ameliorated most of the HSVLF-induced metabolic perturbations [87]. However, dietary MUFA alone was not as effective as a fat source containing both MUFA and PUFA to reduce the hypercholesterolemic effects of the HSVLF diet. Liver-specific Scd1-deficient mice fed the HSVLF diet also developed loss of body weight, hypoglycemia and depleted hepatic glycogen, but only modest hypercholesterolemia and none of the cholestasis-like phenotypes observed in whole-body Scd1-deficient mice [78].
These findings emphasize two important roles for SCD1 during very low-fat, high-carbohydrate feeding conditions. 1) Lack of SCD1 results in an increased requirement for dietary unsaturated fat to compensate for reduced de novo MUFA synthesis. 2) Endogenous MUFA synthesis is essential during dietary unsaturated fat insufficiency and influences the dietary requirement of PUFA. This model is consistent with the observation that hepatic Scd1 and MUFA synthesis are repressed by dietary PUFA intake, which may have evolved as a mechanism to maintain cellular unsaturated fatty acid balance [61]. Studies by Chakravarthy et al. in mice with liver-specific deletion of FAS have provided additional evidence for the importance of de novo fatty acid synthesis [89]. When fed a high-carbohydrate diet without fat or subjected to prolonged fasting, these mice developed hypoglycemia and fatty liver coincident with reduced expression of PPARα target genes. These phenotypes did not occur in mice fed a standard chow diet and were corrected by administration of a potent PPARα agonist, suggesting that new fat synthesized by FAS is the preferred endogenous activator of PPARα [89]. Since SCD1 is downstream of FAS and converts FAS-derived saturated fatty acids into MUFA, it is possible that impaired hepatic MUFA synthesis is responsible for many of the metabolic phenotypes elicited by hepatic deficiency of either SCD1 or FAS.
4.4 The relation of human SCD1 to carbohydrate-induced obesity
Similar to mouse SCD1, human SCD1 is expressed in several tissues, is highest in liver and adipose, and shares 94.1% amino acid identity with mouse SCD1 [90]. The promoter of human SCD1 has been cloned and characterized [91]. Transcription factor binding sites for SREBP, NF-Y, and the PUFA response element were present in the human SCD1 promoter similar to the mouse Scd1 promoter. Most human studies have inferred SCD activity via the ratio of monounsaturated to saturated fatty acids in plasma lipids, as discussed below. However, Chong et al. have recently shown that a short-term feeding of a high-carbohydrate diet elicits a parallel increase in both DNL and hepatic SCD activity, as measured by conversion of [2H]-palmitic acid to [2H]-palmitoleic acid in VLDL-triglyceride [94]. These data strongly suggest that human SCD1 expression and hepatic lipid synthesis respond similarly to dietary carbohydrate as found in rodent models.
Sampath and Ntambi recently reviewed the use of the SCD plasma desaturation index (18:1/18:0 or 16:1/16:0) in humans as a surrogate marker for SCD activity and its association with metabolic outcomes [92]. Although this plasma index does not allow for the distinction between liver and adipose SCD1 activity, it has been shown to correlate well with plasma triglyceride levels [76, 85, 93, 94] and body weight [76, 95]. A 4–6 week-long high-carbohydrate diet increased the 18:1/18:0 ratio and this explained 44% of the variance in the plasma triglyceride response [85]. Additionally, a short-term 3-day low-fat, high-carbohydrate feeding elicited a parallel increase in the desaturation index, hepatic lipogenesis, and plasma triglycerides [94]. An elevated SCD desaturation index has also been shown to correlate well with the dyslipidemia observed in familial combined hyperlipidemia, potentially due to genetic variations that affect SCD1 activity [93]. Mangravite et al. have recently shown that adipose levels of SCD1 mRNA are lowered independently by both acute weight loss as well as isocaloric reduction in carbohydrate intake [96]. Furthermore, adipose SCD1 levels correlated with plasma triglycerides, but this was independent of carbohydrate intake and the plasma SCD desaturation index [96]. This suggests that modulation of both hepatic and adipose SCD1 levels may influence the plasma triglyceride response via different mechanisms.
As discussed in sections 2 and 3, dietary conditions that promote increased insulin secretion will result in high SCD1 activity, which is associated with a metabolic state favoring hepatic triglyceride accumulation and an expansion of adipose triglyceride stores. Changes in SCD1 activity may influence obesity in two ways. In both human and animal models, elevated levels of SCD1 have been suggested to influence the partitioning of fatty acids towards storage and away from oxidation [15–17]. However, elevated hepatic SCD1 activity and MUFA production may also lead to increased synthesis and transport of VLDL triglycerides to peripheral tissues (Figure 2). In human studies, dietary carbohydrates (primarily sugars) have consistently been shown to increase both hepatic de novo lipogenesis and fasting plasma triglycerides, but the relative contribution of increased VLDL triglyceride production and clearance to the elevated plasma triglyceride phenotype is unresolved (reviewed in [11, 42]). Interestingly, Chaput et al. recently reported a novel interaction between dietary composition and insulin secretion in which patients predisposed to acute hypersecretion of insulin gained the most weight, especially among those consuming low-fat diets [13]. Since insulin has many physiological effects occurring in a variety of tissues, the effects of hyperinsulinemia on the development of obesity are likely not limited to altered hepatic triglyceride production.
5. Conclusion
High-carbohydrate diets, especially those enriched in simple carbohydrates, increase hepatic de novo lipogenesis coincident with elevated hepatic Scd1 expression. This involves pro-lipogenic effects of increased circulating glucose, fructose and insulin to promote activation of SREBP-1, ChREBP, and LXR. Whole-body or liver-specific deletion of Scd1 in mice dramatically reduces the lipogenic effects of dietary carbohydrate by decreasing MUFA production and preventing the upregulation of lipogenic gene expression. However, several key questions remain unanswered in relation to the role of SCD1 in metabolism. First, does oleate or another product of SCD1 directly activate SREBP processing? If so, would increased hepatic Scd1 expression in the absence of elevated blood glucose and insulin be sufficient to promote SREBP processing and de novo lipogenesis? Alternatively, the impaired SREBP processing and lipogenesis may be the indirect result of energy deficiency or altered membrane composition. The role of extrahepatic SCD1 in carbohydrate-induced adiposity also needs to be investigated. Since liver-specific Scd1-deficient mice are protected from high-carbohydrate, but not high-fat diet-induced obesity, Scd1 expression in extrahepatic tissues such as the adipose, muscle, brain, and skin may also play a pivotal metabolic role. However, the blunted carbohydrate-induced lipogenesis in liver-specific Scd1-deficient mice suggest that these tissues cannot compensate for local hepatic depletion of MUFA. Together, the current body of literature suggests that high-carbohydrate diets act through modulation of hepatic SCD1 activity to increase the conversion of carbohydrates to MUFA, which enhance hepatic triglyceride synthesis and secretion. Presumably, this rise in hepatic triglyceride secretion promotes the storage of carbohydrates as triglycerides in the adipose tissue, contributing to the development of obesity.
Acknowledgments
We would like to thank Adam Steinberg at the University of Wisconsin Department of Biochemistry Media Center for his artistic contributions to the figures. This work was supported by NIH grant RO1DK-62388 (To J.M.N.). M.T.F. was supported by a postdoctoral fellowship from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–52. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–7. doi: 10.1097/01.mol.0000174153.53683.f2. [DOI] [PubMed] [Google Scholar]
- 4.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–6. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368:1–19. doi: 10.1016/j.cca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Krebs-Smith SM, Kris-Etherton P. How does MyPyramid compare to other population-based recommendations for controlling chronic disease? J Am Diet Assoc. 2007;107:830–7. doi: 10.1016/j.jada.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Reedy J, Krebs-Smith SM. A comparison of food-based recommendations and nutrient values of three food guides: USDA’s MyPyramid, NHLBI’s Dietary Approaches to Stop Hypertension Eating Plan, and Harvard’s Healthy Eating Pyramid. J Am Diet Assoc. 2008;108:522–8. doi: 10.1016/j.jada.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Pagoto SL, Griffith JA, Merriam PA, Ockene IS, Hafner AR, Olendzki BC. A dietary quality comparison of popular weight-loss plans. J Am Diet Assoc. 2007;107:1786–91. doi: 10.1016/j.jada.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A TO Z weight loss study. Int J Obes (Lond) 2008;32:985–91. doi: 10.1038/ijo.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellerstein MK. Carbohydrate-induced hypertriglyceridemia: modifying factors and implications for cardiovascular risk. Curr Opin Lipidol. 2002;13:33–40. doi: 10.1097/00041433-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Krauss RM. Dietary and genetic effects on low-density lipoprotein heterogeneity. Annu Rev Nutr. 2001;21:283–95. doi: 10.1146/annurev.nutr.21.1.283. [DOI] [PubMed] [Google Scholar]
- 13.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87:303–9. doi: 10.1093/ajcn/87.2.303. [DOI] [PubMed] [Google Scholar]
- 14.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–56. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–93. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–8. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 20.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- 22.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 23.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 25.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spady DK, Woollett LA, Dietschy JM. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr. 1993;13:355–381. doi: 10.1146/annurev.nu.13.070193.002035. [DOI] [PubMed] [Google Scholar]
- 27.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 28.Hayes KC, Khosla P. Dietary fatty acid thresholds and cholesterolemia. FASEB J. 1992;6:2600–2607. doi: 10.1096/fasebj.6.8.1592210. [DOI] [PubMed] [Google Scholar]
- 29.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87:1558S–1561S. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- 30.Buchholz AC, Schoeller DA. Is a calorie a calorie? Am J Clin Nutr. 2004;79:899S–906S. doi: 10.1093/ajcn/79.5.899S. [DOI] [PubMed] [Google Scholar]
- 31.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–37. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 32.Galgani J, Aguirre C, Diaz E. Acute effect of meal glycemic index and glycemic load on blood glucose and insulin responses in humans. Nutr J. 2006;5:22. doi: 10.1186/1475-2891-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–22. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 34.Hallfrisch J, Facn Behall KM. Mechanisms of the effects of grains on insulin and glucose responses. J Am Coll Nutr. 2000;19:320S–325S. doi: 10.1080/07315724.2000.10718967. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 36.Prentki M, Tornheim K, Corkey BE. Signal transduction mechanisms in nutrient-induced insulin secretion. Diabetologia. 1997;40(Suppl 2):S32–41. doi: 10.1007/s001250051395. [DOI] [PubMed] [Google Scholar]
- 37.Gravena C, Mathias PC, Ashcroft SJ. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J Endocrinol. 2002;173:73–80. doi: 10.1677/joe.0.1730073. [DOI] [PubMed] [Google Scholar]
- 38.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–94. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans. 2007;35:1180–6. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- 40.Liang Y, Matschinsky FM. Mechanisms of action of nonglucose insulin secretagogues. Annu Rev Nutr. 1994;14:59–81. doi: 10.1146/annurev.nu.14.070194.000423. [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–52. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Chong MF, Fielding BA, Frayn KN. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc. 2007;66:52–9. doi: 10.1017/S0029665107005290. [DOI] [PubMed] [Google Scholar]
- 43.Timlin MT, Parks EJ. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am J Clin Nutr. 2005;81:35–42. doi: 10.1093/ajcn/81.1.35. [DOI] [PubMed] [Google Scholar]
- 44.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–91. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudgins LC, Seidman CE, Diakun J, Hirsch J. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am J Clin Nutr. 1998;67:631–9. doi: 10.1093/ajcn/67.4.631. [DOI] [PubMed] [Google Scholar]
- 46.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–46. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–20. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 48.Koo HY, Wallig MA, Chung BH, Nara TY, Cho BH, Nakamura MT. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta. 2008;1782:341–8. doi: 10.1016/j.bbadis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 50.Behall KM, Howe JC. Effect of long-term consumption of amylose vs amylopectin starch on metabolic variables in human subjects. Am J Clin Nutr. 1995;61:334–40. doi: 10.1093/ajcn/61.2.334. [DOI] [PubMed] [Google Scholar]
- 51.Behall KM, Scholfield DJ, Canary J. Effect of starch structure on glucose and insulin responses in adults. Am J Clin Nutr. 1988;47:428–32. doi: 10.1093/ajcn/47.3.428. [DOI] [PubMed] [Google Scholar]
- 52.Behall KM, Scholfield DJ, Yuhaniak I, Canary J. Diets containing high amylose vs amylopectin starch: effects on metabolic variables in human subjects. Am J Clin Nutr. 1989;49:337–44. doi: 10.1093/ajcn/49.2.337. [DOI] [PubMed] [Google Scholar]
- 53.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364:778–85. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- 54.Scribner KB, Pawlak DB, Ludwig DS. Hepatic steatosis and increased adiposity in mice consuming rapidly vs. slowly absorbed carbohydrate. Obesity (Silver Spring) 2007;15:2190–9. doi: 10.1038/oby.2007.260. [DOI] [PubMed] [Google Scholar]
- 55.Scribner KB, Pawlak DB, Aubin CM, Majzoub JA, Ludwig DS. Long-term effects of dietary glycemic index on adiposity, energy metabolism, and physical activity in mice. Am J Physiol Endocrinol Metab. 2008;295:E1126–31. doi: 10.1152/ajpendo.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–50. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 58.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–51. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 59.Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest. 2008;118:956–64. doi: 10.1172/JCI34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denechaud PD, Girard J, Postic C. Carbohydrate responsive element binding protein and lipid homeostasis. Curr Opin Lipidol. 2008;19:301–6. doi: 10.1097/MOL.0b013e3282ffafaa. [DOI] [PubMed] [Google Scholar]
- 61.Kim HJ, Miyazaki M, Ntambi JM. Dietary cholesterol opposes PUFA-mediated repression of the stearoyl-CoA desaturase-1 gene by SREBP-1 independent mechanism. J Lipid Res. 2002;43:1750–1757. doi: 10.1194/jlr.m100433-jlr200. [DOI] [PubMed] [Google Scholar]
- 62.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–98. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 64.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 2007;13:171–80. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- 66.Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mrnas. J Biol Chem. 1999;274:25892–25898. doi: 10.1074/jbc.274.36.25892. [DOI] [PubMed] [Google Scholar]
- 67.Xu J, Teran-Garcia M, Park JH, Nakamura MT, Clarke SD. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J Biol Chem. 2001;276:9800–7. doi: 10.1074/jbc.M008973200. [DOI] [PubMed] [Google Scholar]
- 68.Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–54. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J, Christian B, Jump DB. Regulation of rat hepatic L-pyruvate kinase promoter composition and activity by glucose, n-3 polyunsaturated fatty acids, and peroxisome proliferator-activated receptor-alpha agonist. J Biol Chem. 2006;281:18351–62. doi: 10.1074/jbc.M601277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 71.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–44. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–19. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 73.Ntambi JM. Dietary regulation of stearoyl-CoA desaturase 1 gene expression in mouse liver. J Biol Chem. 1992;267:10925–10930. [PubMed] [Google Scholar]
- 74.Bene H, Lasky D, Ntambi JM. Cloning and characterization of the human stearoyl-CoA desaturase gene promoter: transcriptional activation by sterol regulatory element binding protein and repression by polyunsaturated fatty acids and cholesterol. Biochem Biophys Res Commun. 2001;284:1194–8. doi: 10.1006/bbrc.2001.5102. [DOI] [PubMed] [Google Scholar]
- 75.Landschulz KT, Jump DB, MacDougald OA, Lane MD. Transcriptional control of the stearoyl-CoA desaturase-1 gene by polyunsaturated fatty acids. Biochem Biophys Res Commun. 1994;200:763–8. doi: 10.1006/bbrc.1994.1516. [DOI] [PubMed] [Google Scholar]
- 76.Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, Daubert JC, Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2007 doi: 10.1016/j.numecd.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 78.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic Stearoyl-CoA Desaturase-1 Deficiency Protects Mice from Carbohydrate-Induced Adiposity and Hepatic Steatosis. Cell Metab. 2007;6:484–96. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 79.Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J Biol Chem. 2004;279:35017–35024. doi: 10.1074/jbc.M405327200. [DOI] [PubMed] [Google Scholar]
- 80.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol. 2005;288:E599–607. doi: 10.1152/ajpendo.00439.2004. [DOI] [PubMed] [Google Scholar]
- 82.Lee SH, Dobrzyn A, Dobrzyn P, Rahman SM, Miyazaki M, Ntambi JM. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J Lipid Res. 2004;45:1674–1682. doi: 10.1194/jlr.M400039-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Dobrzyn A, Dobrzyn P, Miyazaki M, Ntambi JM. Polyunsaturated fatty acids do not activate AMP-activated protein kinase in mouse tissues. Biochem Biophys Res Commun. 2005;332:892–896. doi: 10.1016/j.bbrc.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 84.Binczek E, Jenke B, Holz B, Gunter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–18. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 85.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 86.Blasiole DA, Davis RA, Attie AD. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol Biosyst. 2007;3:608–19. doi: 10.1039/b700706j. [DOI] [PubMed] [Google Scholar]
- 87.Flowers MT, Groen AK, Oler AT, Keller MP, Choi Y, Schueler KL, Richards OC, Lan H, Miyazaki M, Kuipers F, Kendziorski CM, Ntambi JM, Attie AD. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J Lipid Res. 2006;47:2668–80. doi: 10.1194/jlr.M600203-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, Attie AD. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genomics. 2008;33:361–72. doi: 10.1152/physiolgenomics.00139.2007. [DOI] [PubMed] [Google Scholar]
- 89.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–22. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332:735–42. doi: 10.1016/j.bbrc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 91.Zhang L, Ge L, Tran T, Stenn K, Prouty SM. Isolation and characterization of the human stearoyl-CoA desaturase gene promoter: requirement of a conserved CCAAT cis-element. Biochem J. 2001;357:183–93. doi: 10.1042/0264-6021:3570183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sampath H, Ntambi JM. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidology. 2008;3:163–173. [Google Scholar]
- 93.Mar-Heyming R, Miyazaki M, Weissglas-Volkov D, Kolaitis NA, Sadaat N, Plaisier C, Pajukanta P, Cantor RM, de Bruin TW, Ntambi JM, Lusis AJ. Association of stearoyl-CoA desaturase 1 activity with familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 2008;28:1193–9. doi: 10.1161/ATVBAHA.107.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87:817–23. doi: 10.1093/ajcn/87.4.817. [DOI] [PubMed] [Google Scholar]
- 95.Warensjo E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis. 2006;16:128–36. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Mangravite LM, Dawson K, Davis RR, Gregg JP, Krauss RM. Fatty acid desaturase regulation in adipose tissue by dietary composition is independent of weight loss and is correlated with the plasma triacylglycerol response. Am J Clin Nutr. 2007;86:759–67. doi: 10.1093/ajcn/86.3.759. [DOI] [PubMed] [Google Scholar]