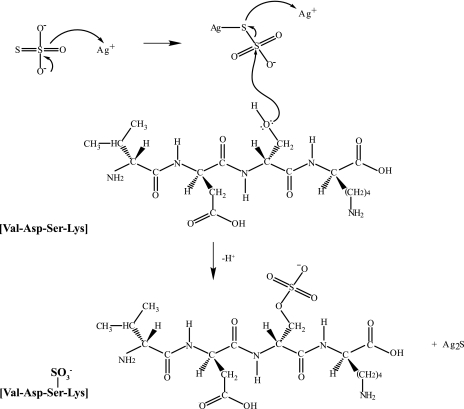
Fig. 6.
Proposed mechanism for sulfation of hydroxylated amino acids in silver-stained gels. Sodium thiosulfate reduces silver ions to form a reactive metallic intermediate that is then subject to nucleophilic attack by the lone electron pairs of hydroxylated residues (shown here for Ser). The thiosulfate intermediate can reduce a second silver ion proximal to acidic residues to produce Ag2S necessary for visualizing the gel-separated protein and liberate a sulfated peptide.