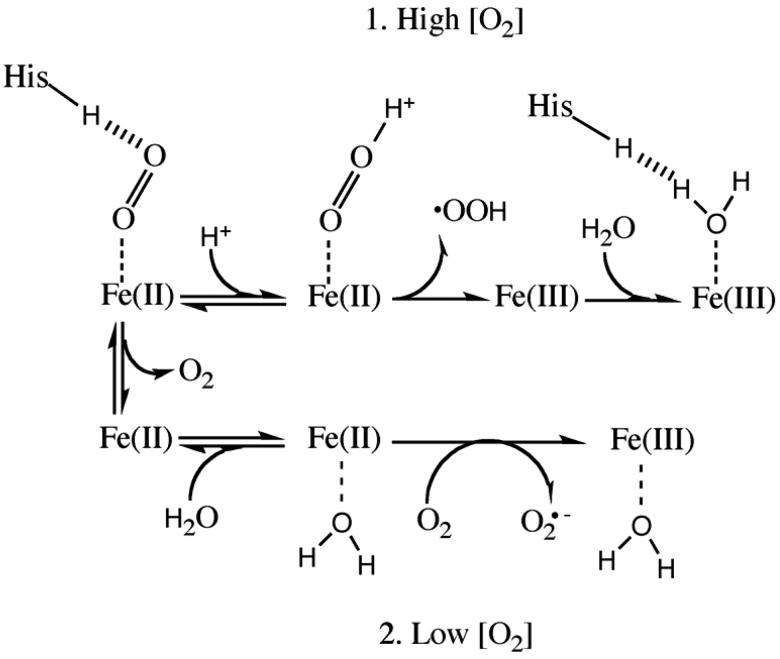
Figure 1.
Mechanism of iron oxidation in heme. The heme iron can be oxidized in two mechanisms: when the concentration of O2 is high (the top method) or low (the bottom method) (Adapted from 14). Under high concentrations of O2 (1), a hydronium molecule bonds with O2 and the ligand leaves as a neutral superoxide radical. A water can then hydrogen bond with the distal His. Under low concentrations of O2 (2), a water molecule can displace the ligand. Reentry of O2 can remove an electron from the heme iron in which the coordinated water facilitates the removal of the iron electron to O2. The ligand leaves the heme pocket as superoxide anion radical. In both scenarios the iron heme is oxidized to Fe(III).