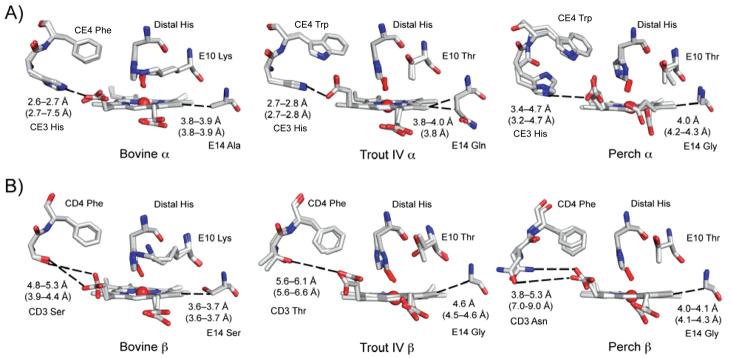
Figure 5.
Structural differences in the heme pocket of bovine, trout IV, and perch Hb. Structural differences of heme pocket residues are shown for the bovine Hb at pH 8.5, the trout IV Hb at pH 6.3, and the perch Hb at pH 8.0 are shown for the A) α and B) β subunits. The CO-bound bovine at pH 8.5 was overlaid onto the trout IV and perch Hbs to place the CO (in the red stick model). The residues are labeled accordingly and contact distances to the heme propionates are listed in black for the structures shown and in parentheses calculated using the structures at pH 5.7 for all three Hbs.