Abstract
The protein kinase mTOR (mammalian target of rapamycin) is a critical regulator of cellular metabolism, growth, and proliferation. These processes contribute to tumor formation, and many cancers are characterized by aberrant activation of mTOR. Although activating mutations in mTOR itself have not been identified, deregulation of upstream components that regulate mTOR are prevalent in cancer. The prototypic mechanism of mTOR regulation in cells is through activation of the PI3K/Akt pathway, but mTOR receives input from multiple signaling pathways. This review will discuss Akt-dependent and independent mechanisms of mTOR regulation in response to mitogenic signals, as well as its regulation in response to energy and nutrient-sensing pathways. Preclinical and clinical studies have demonstrated that tumors bearing genetic alterations that activate mTOR are sensitive to pharmacologic inhibition of mTOR. Elucidation of novel pathways that regulate mTOR may help identify predictive factors for sensitivity to mTOR inhibitors and could provide new therapeutic targets for inhibiting the mTOR pathway in cancer. This review will also highlight pharmacologic approaches that inhibit mTOR via activation of the AMP-activated protein kinase (AMPK), an important inhibitor of the mTOR pathway and an emerging target in cancer.
Keywords: mTOR, cancer, Akt, AMPK
1. Introduction
The serine-threonine kinase mTOR is a master regulator of protein synthesis, and plays important roles in other biological processes that support cell growth and survival, such as angiogenesis and autophagy. mTOR exists in two functionally distinct complexes in cells, namely mTORC1 and mTORC2. mTORC1 is composed of mTOR, Raptor, mLST8, and PRAS40, and is sensitive to inhibition by the macrolide antibiotic rapamycin. Importantly, mTORC1 activates S6K1 (p70 ribosomal protein S6 kinase) and inactivates 4E-BP1 (eIF4E binding protein 1), which promotes protein translation and cell growth. Conversely, mTORC2 is composed of mTOR, Rictor, Sin1, and mLST8. Although originally reported to be insensitive to rapamycin, long-term treatment of mammalian cells with rapamycin indirectly inhibits mTORC2 [1]. The role of mTORC2 in regulating cellular processes is not well understood. However, mTORC2 can regulate the assembly of the actin cytoskeleton in response to mitogenic signals through phosphorylation and activation of PKCα, a member of the AGC family of serine-threonine kinases [2, 3]. mTORC2 also phosphorylates and activates another member of the AGC kinase family, Akt [4]. Because Akt promotes cell proliferation and survival and inhibits apoptosis, activation of Akt by mTORC2 could be another important mechanism by which mTOR promotes tumorigenesis.
2. Canonical pathway of mTOR (Akt-dependent) regulation
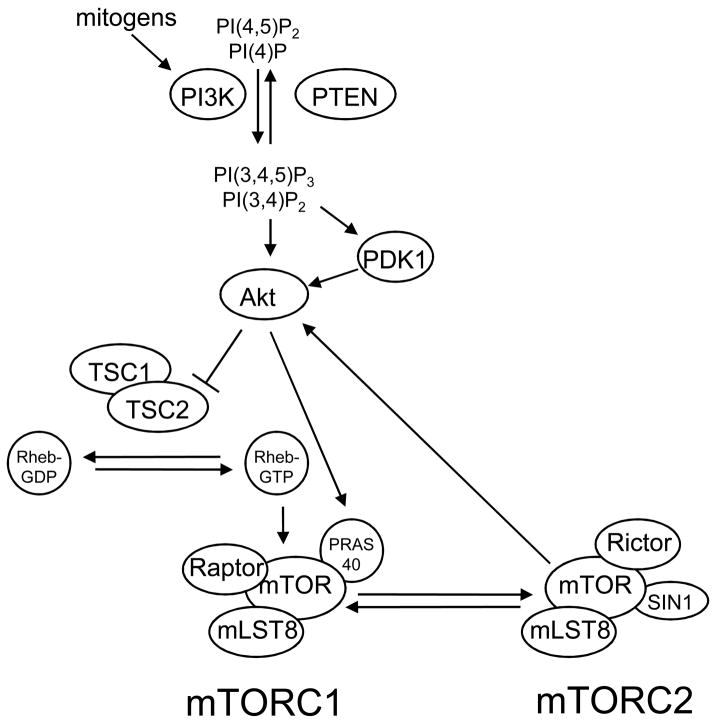
mTOR is regulated by mitogen-responsive signaling pathways and pathways that signal the availability of intracellular energy and nutrients such as amino acids. The prototypic mechanism for mTOR regulation by mitogenic signals is activation by the PI3K/Akt pathway (Figure 1). PI3K is a lipid kinase that is activated by multiple mechanisms, for example, binding of growth factors to receptor tyrosine kinases, activation of G-protein-coupled receptors, as well as by oncogenes such as Ras. Once activated, PI3K phosphorylates phosphoinositides at the D3 position, generating the biologically active lipids, phosphatidylinositol-3,4-bisphosphate (PI(3,4)P2) and phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5,)P3). The tumor suppressor and lipid phosphatase PTEN opposes the activity of PI3K by dephosphorylating phosphoinositides at this site. PI(3,4,5,)P3 binds to the pleckstrin homology (PH) domain of the serine-threonine kinase Akt, promoting its translocation to the cell membrane [5, 6]. Akt is then activated by sequential phosphorylation at T308 and S473, residues that are located within the activation loop and C-terminal hydrophobic motif of Akt, respectively. Phosphorylation at T308 is mediated by PDK-1 (3′phosphoinositide-dependent kinase 1), which itself is activated by the binding of PI(3,4,5)P3 to its PH domain and subsequent translocation to the cell membrane [7]. There are many kinases that are capable of phosphorylating Akt at S473. These include PDK-1 [8], integrin-linked kinase (ILK) or an ILK-associated kinase [9, 10], DNA-dependent protein kinase (DNA-PK) [11, 12], and Akt itself [13]. However, the strongest data supports mTORC2, which can phosphorylate Akt at S473 in vitro and in vivo, thereby indicating that mTOR can act as both a substrate and effector of the Akt signaling pathway.
Figure 1. Prototypic mechanism of mTOR regulation by the PI3K/Akt pathway.
The lipid kinase PI3K is activated in response to mitogenic signals and phosphorylates the phosphoinositides PI(4)P and PI(4,5)P2 at their D3 position, generating PI(3,4)P2 and PI(3,4,5)P3, respectively. The tumor suppressor PTEN opposes this activity of PI3K. PI(3,4)P2 and PI(3,4,5)P3 bind to Akt and PDK1, promoting their translocation to the cell membrane. Akt is then activated by sequential phosphorylation of T308 and S473 by PDK1 and mTORC2, respectively. Akt, in turn, activates mTORC1 indirectly by phosphorylation and inactivation of TSC2, which suppresses the activity of the Rheb GTPase, an activator of mTORC1. Akt also directly activates mTORC1 through phosphorylation of PRAS40, a component of mTORC1.
Although Akt has many substrates within the cell, phosphorylation of two substrates, TSC2 and PRAS40, leads to activation of mTOR. Akt indirectly activates mTORC1 by direct phosphorylation of the tumor suppressor TSC2 at S939 and T1462 [14, 15]. TSC2 forms a heterodimeric complex with TSC1, and phosphorylation of TSC2 at these sites inhibits the GAP (GTPase-activating protein) activity of this complex. Because TSC2 suppresses the activity of the Ras-related GTPase Rheb, a selective activator of mTORC1, inhibition of TSC2 by Akt results in activation of mTORC1 [16]. The importance of TSC2 in regulating mTOR is perhaps best demonstrated in patients with tuberous sclerosis (TSC). Germline mutations in TSC2 and TSC1 occur in TSC, which causes the growth of benign tumors called hamartomas in many organs. Additionally, somatic mutations in tuberous sclerosis genes occur in lymphangioleiomyomatosis (LAM), a pulmonary proliferative disorder associated with renal angiomyolipomas [17]. Lesions that develop in these diseases are characterized by constitutive activation of the mTOR pathway [18, 19]. Preclinical and clinical studies demonstrated that mTOR inhibitors, such as rapamycin and the rapamycin analogue CCI-779, inhibit tumor growth in mouse models of TSC as well as TSC patients [20–22]. These studies demonstrate the importance of the tuberous sclerosis complex in regulating the mTOR pathway, and show that inhibition of mTOR is sufficient to reverse or ameliorate many clinical manifestations of TSC.
Akt also activates mTORC1 by a TSC2-independent mechanism. Studies performed using mass spectrometry demonstrated that Akt directly phosphorylates PRAS40 (proline-rich Akt substrate 40kDa), a protein that associates with mTORC1 [23]. Akt-mediated phosphorylation of PRAS40 attenuates its inhibitory effect on mTORC1 [24, 25]. Although the mechanism by which PRAS40 interacts with mTORC1 is controversial, PRAS40 inhibits mTORC1 independently of TSC2 because overexpression of PRAS40 in 293T cells transduced with TSC2 shRNA suppresses S6K1 phosphorylation. Overexpression of PRAS40 in cancer cells can inhibit mTORC1 and cell proliferation, but inactivation of PRAS40 has not been reported in cancers with elevated mTOR activity. In addition to phosphorylation of TSC2 and PRAS40, Akt can also directly phosphorylate mTOR at S2448 in response to insulin stimulation [26]. However, mutation of this residue to alanine, which prevents its phosphorylation, does not affect downstream signaling to two substrates of mTORC1, S6K1 and 4E-BP1 [27]. Therefore, it seems unlikely that phosphorylation of mTOR at S2448 is required for Akt-mediated activation of mTORC1. Collectively, these studies demonstrate that the PI3K/Akt pathway propagates mitogenic signals to the mTOR pathway by TSC2-dependent and independent mechanisms.
3. Akt-independent mechanisms of mTOR regulation
3.1. Akt-independent regulation of mTOR by mitogen-responsive pathways
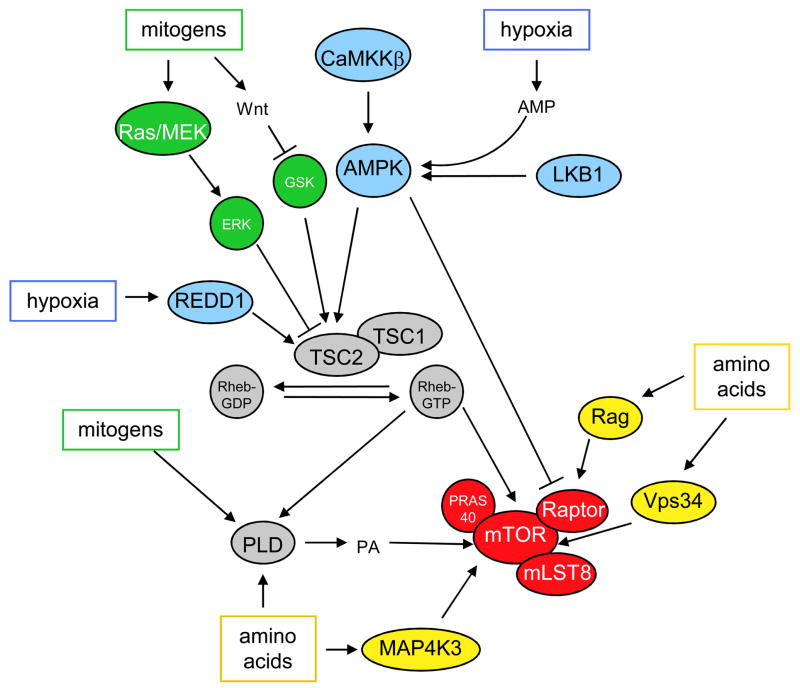
mTOR is also activated by mitogenic signals through activation of the Ras/MEK/ERK pathway (Figure 2). Analysis of brain lesions from TSC patients showed that constitutive activation of ERK frequently occurs in lesions that retain wt TSC1 or TSC2 alleles [28, 29]. This suggested that Erk might post-translationally inhibit TSC function, thereby activating mTOR and promoting tumorigenesis. Studies performed using mass spectrometry and Scansite, an internet-based bioinformatics platform that analyzes protein phosphorylation motifs, identified S664 and S540 as putative ERK phosphorylation sites in TSC2 [30]. A constitutively active MEK1 mutant induced TSC2 phosphorylation, which was markedly reduced by co-transfection with a non-phosphorylatable S664A/S540A TSC2 mutant. Phosphorylation of TSC2 by ERK promoted dissociation of the tuberous sclerosis complex and attenuated TSC2-mediated inhibition of mTOR in cells. Inhibition of TSC2 by Erk also promoted tumorigenesis in vitro and in vivo. Tsc2ang1 sarcoma cells, a cell line derived from TSC2+/− mice, contain constitutively active Erk and form tumors in nude mice. Retroviral infection of these cells with the S664A/S540A TSC2 mutant significantly inhibited soft agar colony formation in vitro and greatly reduced tumor growth in nude mice. Inhibition of tumorigenesis correlated with mTOR pathway inhibition in these cells. Collectively, these studies demonstrate the importance of the Ras-MAPK signaling pathway in promoting mTOR-mediated tumorigenesis through Erk-dependent phosphorylation and inactivation of TSC2.
Figure 2. Akt-independent mechanisms of mTOR regulation.
The mTOR pathway is regulated by mitogen-responsive signaling pathways (shown in green), as well as by pathways that signal the availability of nutrients, such as amino acids (shown in yellow), and intracellular energy (shown in blue). Mitogens activate mTOR independently of Akt through the Ras/Mek/ERK pathway, the Wnt signaling pathway, and through activation of phospholipase D. Amino acids also activate mTOR through multiple mechanisms: activation of Vps34, MAP4K3, the Rag family of GTPases, and phospholipase D. However, the mechanisms by which some of these proteins activate mTOR are not well understood, and there may be crosstalk between these pathway components. Conversely, conditions that deplete intracellular energy, such as hypoxia, inhibit the mTOR pathway. One mechanism by which this occurs is through activation of AMPK. AMPK is activated by increases in intracellular AMP, as well as by the upstream kinases, LKB1 and CaMKKβ. AMPK inhibits the mTOR pathway directly through phosphorylation of Raptor and indirectly through phosphorylation and activation of TSC2. Although hypoxia can inhibit the mTOR pathway through activation of AMPK, it also inhibits mTOR through increased expression of the hypoxia-inducible-factor-1 target gene, REDD1. The tuberous sclerosis complex TSC1/TSC2 and Rheb GTPase (shown in grey) integrate signals from mitogen-responsive and energy and nutrient-sensing pathways to regulate mTORC1 (shown in red).
3.2. Akt-independent regulation of mTOR by energy-sensing pathways
In addition to mitogenic signals, the mTOR pathway is responsive to changes in the energy status of the cell, i.e., conditions that deplete intracellular energy inhibit mTOR. One mechanism by which this occurs is through inhibition of mTORC1 by the master metabolic regulator AMP-activated protein kinase (AMPK). AMPK is a heterotrimeric protein comprised of an alpha catalytic and beta and gamma regulatory subunits. Conditions that deplete intracellular energy activate AMPK by direct binding of AMP to tandem repeats of crysthionine-β synthase (CBS) domains located within the gamma (regulatory) subunit [31]. Binding of AMP to this region induces a conformational change in the holoenzyme that prevents PP2C-mediated dephosphorylation of T172, a residue located within the activation loop of AMPK’s catalytic subunit [32]. Phosphorylation at this site is required for activation of AMPK. Therefore, cellular stresses such as nutrient deprivation and hypoxia that increase the intracellular AMP:ATP ratio, activate AMPK.
AMPK is activated by at least two upstream kinases. Under conditions that increase intracellular AMP, LKB1 phosphorylates AMPK at T172 [33–35]. LKB1 is an important tumor suppressor. Germline mutations in LKB1 occur in the hamartomatous syndrome, Peutz-Jeghers Syndrome (PJS), which also predisposes to many types of malignant cancer [36]. Additionally, somatic mutations in LKB1 occur in 30–50% of sporadically occurring lung adenocarcinomas, as well as other histological types of NSCLC [37–39]. Interestingly, immunohistochemical analysis of GI polyps that develop in LKB1-deficient mouse models of PJS showed that mTOR pathway activity is elevated in the epithelium of these polyps [40]. A subsequent study demonstrated that treatment of LKB1-deficient mice with the mTOR inhibitor rapamycin significantly decreased tumor burden [41]. Additionally, NSCLC cells containing mutant LKB1 are resistant to mTOR inhibition and cell death in response to glucose deprivation [42]. Collectively, these studies underscore the importance of the LKB1/AMPK pathway in inhibiting mTORC1.
More recently, CaMKKβ was identified as another kinase that phosphorylates AMPK at T172 [43–45]. Unlike LKB1, activation of AMPK by CaMKKβ occurs by a mechanism that is AMP-independent. CaMKKβ activates AMPK in response to conditions that increase intracellular calcium, for example, in neurons following K+-induced depolarization [43]. Additionally, binding of hormones and cytokines, such as leptin, thrombin, and epinephrine, to Gq-coupled receptors activates AMPK [46–48]. Because stimulation of these receptors results in calcium release, activation of AMPK under these conditions likely occurs by a CaMKKβ-dependent mechanism. However, these studies did not investigate if inhibition of mTORC1 occurred under these conditions. Also, unlike LKB1, CaMKKβ is not ubiquitously expressed, so it may function in a tissue-specific manner to inhibit mTORC1 [49]. Therefore, the importance of the CaMKKβ/AMPK pathway in inhibiting mTOR in cells is unclear. However, a study performed by our group suggested that pharmacologic activation of the CaMKKβ/AMPK pathway played an important role in inhibiting mTOR in NSCLC cells. We showed that treatment of LKB1-mutant NSCLC cell lines with a lipid-based Akt inhibitor activated AMPK by a mechanism that was CaMKKβ-dependent but independent of LKB1 or Akt [50]. Interestingly, PIAs inhibited the mTOR pathway in NSCLC cells even in the absence of Akt inhibition, suggesting that activation of the CaMKKβ/AMPK pathway was a major mechanism by which PIAs inhibited mTORC1. It is unclear if dysregulation of the CaMKKβ/AMPK pathway contributes to activation of mTOR in cancer cells.
AMPK communicates the energy status of the cell to the mTOR pathway by both indirect and direct inhibition of mTORC1 (Figure 2). AMPK inhibits mTOR indirectly by phosphorylating the tumor suppressor TSC2 at S1227 and S1345, which causes its activation [51, 52]. You might want to say that these sites are distinct from Akt/ERK sites. As described previously, TSC2 functions in a heterodimeric complex w/TSC1 to suppress the Ras-related GTPase, Rheb, which is a selective activator of mTORC1. Additionally, AMPK-induced phosphorylation at S1345 primes TSC2 to be phosphorylated at S1341 and S1337 by GSK3β, a component of the canonical Wnt signaling pathway [53]. The coordinated phosphorylation of TSC2 by AMPK and GSK3β is required for maximal activation of TSC2 and inhibition of mTORC1. The fact that Akt, ERK, AMPK, and GSK3β phosphorylate TSC2 on distinct sites highlights the importance of TSC2 in integrating energy-sensing and growth signaling pathways to regulate protein synthesis.
Although TSC2 is an important mediator of AMPK inhibition of mTOR, studies performed in TSC2-deficient MEFS demonstrated that pharmacologic activators of AMPK (discussed in section 4) inhibit mTORC1 even in the absence of TSC2. This suggested that AMPK regulates the mTOR pathway by an additional mechanism that is independent of TSC2. Studies performed using tandem-mass spectrometry identified two putative AMPK phosphorylation sites on Raptor (S722 and S792), a component of mTORC1 [54]. Immunoblotting analysis of AMPKα wt and −/− MEFS confirmed that AMPK can phosphorylate these residues in cells upon stimulation. Phosphorylation of Raptor is an important mechanism by which AMPK regulates mTORC1 because stable transfection of cells with an AA Raptor mutant that cannot be phosphorylated suppressed mTORC1 inhibition by the AMPK activators AICAR and phenformin. The fact that AMPK can regulate mTORC1 in a TSC2 dependent and independent manner shows that the mTOR pathway is highly responsive to changes in intracellular energy.
3.3. Akt-independent regulation of mTOR in response to hypoxia
The mTOR pathway is also inhibited in cells in response to hypoxia by a mechanism that is independent of AMPK, but dependent on the hypoxia-inducible REDD1 gene and TSC1/2 complex (Figure 2). Hypoxia (1% O2) rapidly inhibits basal levels of mTORC1 activity in cells, and prevents insulin and growth factor-induced mTORC1 activation [55]. Although hypoxia can deplete intracellular energy and activate AMPK, hypoxia inhibits mTORC1 independently of AMPK because pretreatment of cells with the AMPK inhibitor Compound C does not prevent hypoxia-induced mTOR inhibition [56]. Similarly, hypoxia inhibits the mTOR pathway even in cells deficient or mutant for LKB1, a kinase that activates AMPK. Conversely, REDD1 is required for hypoxia-induced mTOR inhibition because studies that used genetic approaches to decrease expression of REDD1 in cells showed that the effects of hypoxia on the mTOR pathway were abolished under these conditions [56–58]. REDD1 acts upstream of the TSC1/2 complex because hypoxia does not inhibit mTORC1 in cells deficient for TSC1 or TSC2, even when REDD1 is overexpressed [56]. REDD1 promotes TSC1/2-mediated inhibition of mTORC1 by binding to the protein 14-3-3 [59], an inhibitor of the tuberous sclerosis complex [60–62]. Collectively, these studies have identified the REDD1/TSC1/2 pathway as an important regulator of mTOR in response to hypoxia.
REDD1-mediated inhibition of mTOR in response to hypoxia may prevent tumorigenesis. Loss of REDD1 increased colony formation of MEFs that expressed constitutively active Akt when grown under hypoxic conditions, and greatly increased tumor growth in nude mice injected with these cells subcutaneously [59]. Although these studies demonstrated that mTOR was refractory to hypoxia-induced inhibition in these cells, it is not clear if aberrant activation of the mTOR pathway was responsible for tumor growth in this model. Interestingly, studies that analyzed REDD1 expression in primary breast or invasive prostate carcinomas demonstrated that REDD1 expression was decreased in ~30% of these samples compared to patient-matched normal epithelium [59, 63]. These studies suggest that mTOR inhibition by REDD1 in response to hypoxia might prevent tumorigenesis, and could also suggest that cancers with loss of REDD1 might be sensitive to mTOR pathway inhibition.
3.4. Akt-independent activation of mTOR by amino acids
The mTOR pathway is also sensitive to the availability of nutrients, such as amino acids. Increases in the intracellular levels of amino acids, in particular leucine and isoleucine, induce phosphorylation of the mTORC1 substrates, S6K1 and 4E-BP1. There are multiple mechanisms by which amino acids activate the mTOR pathway (Figure 2). For example, amino acid stimulation of nutrient-deprived cells increases MAP kinase kinase kinase kinase-3 (MAP4K3) activity, which correlates with increased phosphorylation of the mTORC1 substrate, S6K1 [64]. Knockdown of MAP4K3 by siRNA abolishes amino acid-induced phosphorylation of S6K1 in HeLa cells. Although MAP4K3 may be an important mediator of mTORC1 activation in response to amino acids, the mechanism by which MAP4K3 regulates mTORC1 is unclear. The class III PI3K, hVps34, also mediates amino acid stimulation of mTOR. hVps34 activates mTOR by a mechanism that is dependent on PtdIns-3-P generation, but independent of TSC2 or Rheb [65, 66]. In fact, studies in TSC-deficient cells demonstrated that the mTOR pathway is activated in response to amino acids even in the absence of TSC [67]. Interestingly, hVps34 is inhibited by AMPK, so it may integrate signals from both nutrient and energy-sensing pathways to regulate mTOR. Because neither hVps34 nor MAP4K3 directly activate mTOR, additional studies are needed to elucidate other molecular mediators of these pathways.
Amino acid stimulation also increases the GTP-loading and activity of the Rag-GTPases in cells [68]. Recently, proteomic analysis of mammalian cells identified the Rag proteins as binding partners of Raptor, a component of mTORC1. There are four Rag proteins (A-D) in mammalian cells, with substantial sequence similarity between Rag A and B and between Rag C and D [69]. These proteins function as heterodimers. Interestingly, the Rag heterodimers interacted with Raptor in an amino acid-sensitive manner. Binding of the Rag GTPases to Raptor promoted the co-localization of mTORC1 with Rheb, an activator of mTORC1, but did not affect the kinase activity of mTORC1. Moreover, studies performed using cells transfected with constitutively active and inactive Rag mutants demonstrated that the Rag proteins are both necessary and sufficient for amino acid activation of mTOR. However, the Rag proteins are not required for regulation of mTORC1 in response to mitogenic signals or energy deprivation. Because amino acids affect the co-localization of Rheb with mTORC1, rather than its activity, this confirms previous studies that demonstrated that amino acids regulate mTOR independently of the GAP activity of TSC2. Additional studies will need to be performed to determine if the Rag proteins may interact with components of other amino-acid sensing pathways, for example hVps34, and whether aberrations in the Rag proteins are present in cancer.
3.5. Akt-independent regulation of mTOR by Phospholipase D and phosphatidic acid
The Phospholipase D/phosphatidic acid (PLD/PA) lipid-signaling cascade activates mTOR in response to mitogenic signals, as well as amino acid availability (Figure 2). PLD can be activated in response to mitogenic signals by the ARF and Rho family of GTPases [70, 71], conventional protein kinase C isoforms (cPKC) [72], and the Ras-ERK pathway [73, 74]. Adequate levels of intracellular amino acids are also required, because amino acid deprivation inhibits serum-induced PLD activation in cells [75]. Once activated, PLD hydrolyzes membrane phosphatidylcholine, generating choline and phosphatidic acid (PA). NMR and mutagenesis studies demonstrated that PA interacts with the FKBP12-rapamycin binding domain (FRB) of mTOR [76]. Although the mechanism by which PA activates mTOR is unclear, binding of PA to FRB is required because mutation of a critical residue located within this domain, R2109, suppresses PA-mediated mTOR activation. Because PA competes with FKBP12-rapamycin for binding to the FBR, increases in intracellular PA or elevated PLD activity decrease the sensitivity of cells to rapamycin [77, 78]. The relative contribution of PLD to the regulation of mTOR is unknown. However, the fact that PLD activity and expression are elevated in multiple cancer types suggests that it might be a useful target in certain cancers (reviewed in [79]).
Although the PLD/PA pathway was thought to regulate mTOR independently of upstream components in the Akt/mTOR pathway, recent studies demonstrated that PLD can act downstream of the Rheb GTPase [75]. Overexpresion of Rheb or loss of TSC2 increases basal levels of PLD activity. Similarly, activation of PLD in response to mitogens was decreased by knockdown of Rheb or overexpression of TSC2 in cells. Rheb activates PLD directly because in vitro assays using purified Rheb and PLD demonstrated that Rheb binds to and activates PLD in a GTP-dependent manner. Importantly, PLD mediates activation of mTOR by Rheb because knockdown of PLD1 by shRNA significantly inhibited phosphorylation of the mTOR substrate S6K in response to overexpression of Rheb in cells. Collectively, these in vitro studies demonstrate a link between two pathways that regulate mTOR, PLD/PA and TSC2/Rheb, and provide further insight into a mechanism by which Rheb activates mTOR.
4. Targeting Akt-independent pathways that regulate mTOR in cancer
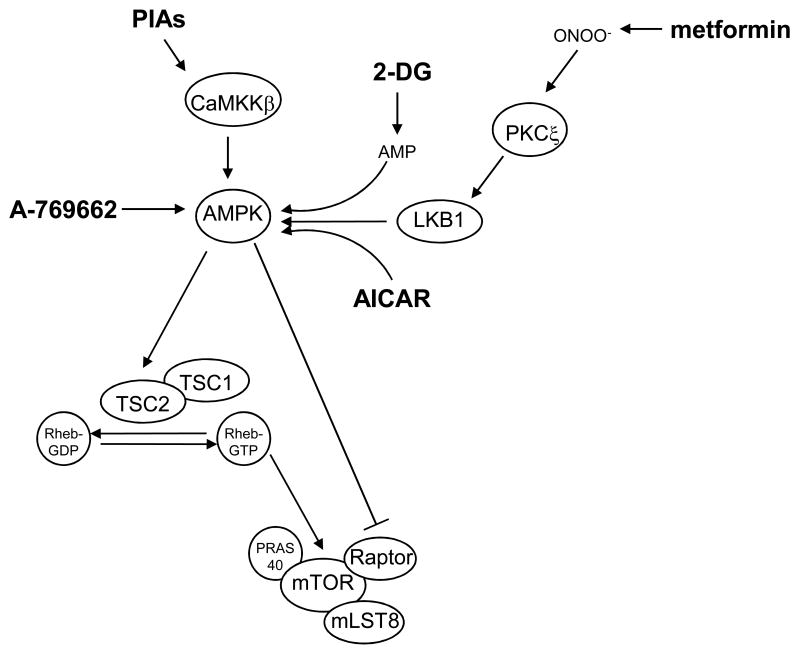
The mTOR pathway promotes tumorigenesis and is an attractive therapeutic target in cancer. Clinical trials indicate that rapamycin, an indirect but specific inhibitor of mTOR, and rapamycin analogues may be effective in the treatment of multiple types of cancer [80–84]. However, the development and application of these drugs as anti-cancer agents may be limited because of inability to achieve sufficient levels in tumors or toxicities. Targeting upstream signaling pathways that regulate mTOR may provide new therapeutic approaches for inhibiting mTOR in cancer. Drugs that target many of the components of these pathways, such as hVps34 and the Rag proteins, have not been identified. However, there are multiple AMPK activators in various stages of preclinical and clinical development. Because AMPK inhibits the mTOR pathway, drugs that activate AMPK may be effective in the treatment of cancer. The activators of AMPK that are best described are metformin, AICAR, 2-DG, PIAs, and A-769662 (Figure 3).
Figure 3. Pharmaceutical approaches to inhibit the mTOR pathway via AMPK.
Because AMPK inhibits mTORC1 by direct and indirect mechanisms, drugs that activate AMPK could be effective in the treatment of cancer. The best-described AMPK activators (shown in bold) are metformin, AICAR, 2-DG, PIAs, and A-769662. Metformin is a biguanide widely prescribed for the treatment of type II diabetes, AICAR is an AMP mimetic, and 2-DG is a glucose analogue. These three drugs activate AMPK by mechanisms that are dependent upon the upstream kinase and tumor suppressor, LKB1. Conversely, the lipid-based Akt inhibitors, PIAs, activate AMPK independently of LKB1 (or Akt, not shown). However, cellular activation of AMPK by PIAs is dependent upon another upstream kinase, CaMKKβ. The thienepyridone A-769662 activates purified AMPK in vitro, and might also activate AMPK directly in cells.
4.1. Metformin
The most clinically developed AMPK activator is the biguanide metformin, which is widely utilized for the treatment of Type II diabetes. The mechanism by which metformin activates AMPK was recently described [85]. Metformin inhibits complex I of the mitochondrial respiratory chain, which results in the generation of reactive nitrogen species (ONOO−). ONOO− then activates AMPK by a mechanism that is PKCζ- and LKB1-dependent. Specifically, ONOO− activates PKCζ, which in turn, phosphorylates LKB1 at S428 [86]. Phosphorylation of LKB1 at this residue is required for its translocation from the nucleus to the cytoplasm and subsequent AMPK activation in response to metformin. FDA-approved doses of metformin activate AMPK in skeletal muscle in patients [87, 88], and long-term treatment is associated with few adverse effects [89]. Interestingly, metformin use in patients is associated with a decrease in cancer incidence, but whether this is related to activation of AMPK is unclear [90].
Activation of AMPK by metformin may be necessary, but not sufficient, to inhibit tumor growth. Studies performed using a panel of breast cancer cell lines demonstrated that metformin treatment significantly inhibited cell proliferation, which was associated with inhibition of S6K and S6 phosphorylation by mTOR in these cells [91]. These effects of metformin were AMPK-dependent because siRNA knockdown of AMPK in cells prevented metformin-induced inhibition of the mTOR pathway and cell growth. Metformin also inhibits cap-dependent translation in cancer cells by an AMPK and mTOR-dependent mechanism [92]. These results suggest that metformin may inhibit cancer cell growth by decreasing protein synthesis. A recent study also evaluated the efficacy of metformin as an anti-cancer agent in a Pten+/−Lkb1fl/+ mouse model [93]. These mice develop cancers of multiple tissue types, such as lymphomas, intestinal polyps, pheochromacytomas, and prostate carcinomas. Pten and LKB1 are both components of pathways that regulate mTOR, and tissues in these mice are characterized by hyperactivity of the mTOR pathway. Although administration of metformin to Pten+/−Llb1fl/+ mice activated AMPK in multiple tissues, it only modestly inhibited tumorigenesis in this mouse model, i.e., it delayed the onset of all tumor types by one month, but did not affect tumor incidence or morphology.
Inhibition of tumorigenesis by metformin and the cellular response to AMPK activation may depend upon the status of tumor suppressor genes such as p53 and LKB1. AMPK directly phosphorylates p53 at S15, which results in its stabilization [94]. Under conditions of nutrient deprivation, stabilization of p53 induces autophagy [95]. This enables cells to survive through degradation and metabolism of cytoplasmic components until extracellular nutrients become available. Therefore, stabilization of p53 by AMPK activators may decrease their efficacy in the treatment of cancer. In fact, studies performed in isogenic HCT-116 p53 wt and −/− xenografts demonstrated that metformin preferentially inhibited the growth of p53-deficient tumors [96]. These results suggest that AMPK activators like metformin may be most effective in the treatment or prevention of cancers that are p53-deficient. Also, because metformin activates AMPK by an LKB1-dependent mechanism, metformin may not be effective in the treatment of LKB1-mutant cancers. Further studies are needed to determine which types of cancer and molecular contexts would be predictive for response to metformin.
4.2. AICAR
5-aminoimidazole-4-carboxamide ribonuclease (AICAR) is an AMP-mimetic that activates AMPK by direct allosteric activation, as well as by promoting its phosphorylation by upstream kinases [97]. LKB1 is an important mediator of AICAR-induced AMPK activation in cells because studies performed using LKB1 wt and −/− MEFs demonstrated that AICAR-induced AMPK phosphorylation was greatly attenuated in the absence of LKB1 [34]. AICAR also inhibits the mTOR pathway by an LKB1/AMPK-dependent mechanism because inhibition of S6K1 and S6 phosphorylation by AICAR was greatly diminished in LKB1-deficient MEFs and LKB1-mutant HeLa cervical carcinoma cells [40]. AMPK activation by AICAR also inhibits the mTOR pathway in vivo. Studies performed using a diabetes-induced model of renal hypertrophy in rats demonstrated that intraperitoneal administration of AICAR activated AMPK in renal cells [98]. This dosing schedule with AICAR also decreased phosphorylation of 4E-BP1 and S6K1, which correlated with inhibition of diabetes-induced renal hypertrophy. Collectively, these studies demonstrate that the AMPK activator AICAR inhibits the mTOR pathway both in vitro and in vivo.
The ability of AICAR to inhibit tumorigenesis was also evaluated in vitro and in vivo. Studies performed using a panel of cancer cell lines demonstrated that AICAR inhibited cell proliferation and arrested cells in S-phase in a dose-dependent manner [99]. The ability of AICAR to inhibit cell proliferation was AMPK-dependent because transfection of cancer cells with a dominant-negative AMPK or pretreatment with the AMPK inhibitor iodotubericidin abolished this effect of AICAR. Additionally, AICAR treatment of C6 glioma cell xenografts decreased tumor weight by ~50%, which correlated with activation of AMPK in these tumors. These studies did not investigate if inhibition of the mTOR pathway contributed to the anti-proliferative effects of AICAR.
Conversely, AICAR may prevent the death of cancer cells that have increased dependence on glucose for survival. For example, cancer cells that express constitutively-active Akt have a high glycolytic rate and die in response to glucose deprivation [100]. Studies performed using Akt-transformed glioblastoma cells demonstrated that AICAR protected these cells from death in response to glucose withdrawal [101]. The protective effects of AICAR were AMPK-dependent because stable expression of dominant-negative AMPK impaired the ability of AICAR to prevent cell death in response to glucose withdrawal. However, this was not due to inhibition of the mTOR pathway because the mTOR inhibitor rapamycin did not protect cancer cells from death under these conditions. These studies suggest that alterations in cancer cell glucose metabolism may affect the ability of AMPK activators such as AICAR to inhibit tumorigenesis.
Although preclinical studies demonstrate that AICAR can inhibit tumorigenesis, the clinical potential of AICAR is limited due to poor pharmacokinetics and toxicity in patients. Clinical trials using intravenous or oral administration of AICAR to patients demonstrated that the bioavailability of AICAR is less than 5% and its half-life is ~ 2 h [102]. Additionally, AICAR use in patients is associated with significant increases in lactic and uric acid production [102–104]. Therefore, AICAR may be a useful research tool for studying the effects of AMPK activation and mTOR inhibition on tumorigenesis, but it is unlikely to have clinical utility.
4.3. 2-Deoxyglucose (2-DG)
2-DG is a non-hydrolyzable glucose analog that inhibits glycolysis and subsequently activates AMPK by increasing intracellular AMP [51]. 2-DG activates AMPK and inhibits mTOR by an LKB1-dependent mechanism because these effects of 2-DG are greatly attenuated in LKB1-deficient MEFs and LKB1-mutant cancer cells [105, 106]. The modest level of AMPK activation that is observed in LKB1-mutant cells in response to 2-DG is likely mediated by CaMKKβ because pretreatment of LKB1-mutant HeLa cancer cells with the CaMKK-specific inhibitor, STO-609, inhibits 2-DG-induced AMPK activation [44]. 2-DG may have clinical potential because oral administration can produce plasma concentrations of 5 mM in patients [107]. Additionally, because 2-DG is preferentially taken up by cancer cells that have elevated glycolytic activity, treatment with 2-DG could have a high therapeutic index in cancer patients. Multiple Phase I/II clinical trials with 2-DG for the treatment of solid tumors are currently being conducted.
Although 2-DG has been shown to inhibit tumorigenesis in vitro and in vivo, it is unclear if this is due to AMPK activation and inhibition of the mTOR pathway in cancer cells. For example, studies performed using nude mice bearing 143b osteosarcoma or MV522 NSCLC xenografts showed that 2-DG, in combination with adriamycin, significantly decreased tumor growth compared to treatment with adriamycin alone [108]. However, 2-DG was ineffective as a single agent in these studies, and it is unknown if this dosing schedule with 2-DG activated AMPK and inhibited mTOR in vivo. Additionally, 2-DG increases cancer cell death in response to ionizing radiation in vitro and in vivo [109, 110], but this may be due to changes in thiol metabolism [111]. 2-DG also significantly decreased mammary carcinoma incidence and multiplicity and increased tumor latency in a 1-methyl-1-nitrosurea-induced rat model of mammary carcinogenesis [112, 113]. Although inhibition of tumorigenesis by 2-DG did correlate with increased AMPK phosphorylation and decreased mTOR phosphorylation, it is not clear if these effects of 2-DG were required for inhibition of tumorigenesis. Interestingly, 2-DG may be more effective in the treatment of cancers with low levels of mTOR activity. Studies performed using a panel of NSCLC cell lines demonstrated that the cytotoxicity of 2-DG inversely correlated with endogenous levels of phosphorylated Akt and mTOR in cells when treated under hypoxic conditions [114]. Pretreatment of cells with the mTOR inhibitor CCI-779 increased the cytotxoicity of 2-DG under these conditions. Therefore, 2-DG may have clinical utility as a cancer chemotherapeutic, but the role of AMPK activation and mTOR in mediating these effects is unclear.
4.4. Phosphatidylinositol ether lipid analogues (PIAs)
PIAs are lipid-based Akt inhibitors that were rationally designed via molecular modeling to target the PH domain of Akt [115]. These drugs are structurally similar to the phosphoinositide (PI(3,4,5,)P3), which normally binds to the PH domain of Akt in cells. High throughput in vitro kinase assays demonstrated that PIAs also activate purified AMPK [50]. Studies performed in NSCLC cell lines confirmed that PIAs activate AMPK independently of either Akt or LKB1. However, AMPK activation by PIAs requires another upstream kinase of AMPK, CaMKKβ, in cells.
Because PIAs activate AMPK independently of LKB1, they may be effective in the treatment of LKB1-mutant cancers, which are characterized by aberrant activation of the mTOR pathway. In fact, PIA treatment of nude mice bearing LKB1-mutant H157 human NSCLC xenografts significantly inhibited tumor growth, which correlated with AMPK activation in these tumors. AMPK activation contributes to the cytotoxicity of PIAs because transfection of H157 cells with a dominant negative AMPK prevented PIA-induced AMPK activation and decreased PIA cytotoxicity by 50%. The fact that inhibition of tumor growth by PIAs in vivo correlated with induction of AMPK phosphorylation in tumor tissue suggests that AMPK phosphorylation could serve as a useful biomarker of PIA administration. As PIAs independently inhibit Akt and activate AMPK, they are distinct amongst existing cancer chemotherapeutics because they negatively regulate the mTOR pathway through two mechanisms.
4.5. A-769662
The thienepyridone A-769662 was recently identified as an AMPK activator by screening of a chemical library of over 700,000 compounds. In vitro kinase assays performed using a panel of purified kinases demonstrated that A-76962 directly activated AMPK, and did not significantly affect the activity of the other seventy-six kinases tested [116, 117]. Although A-769662 induces AMPK phosphorylation at T172 in cells independently of changes in the intracellular AMP:ATP ratio, the role of upstream kinases in mediating cellular AMPK activation by A-769662 is unclear [117, 118]. A-769662 also activates AMPK in vivo, because oral administration of A-769662 to Pten+/+Lkb1+/+ and Pten+/−Lkb1fl/+ mice activated AMPK in the livers, spleens, and intestines of these mice, and did so with greater potency than metformin [93]. A-769662 may have potential as an anti-cancer agent because it significantly increased tumor latency and decreased tumor incidence in Pten+/−Lkb1fl/+ mice. However, these studies did not investigate if AMPK activation contributed to the ability of A-769962 to inhibit tumorigenesis in these mice, nor did they evaluate if A-769662 inhibited the mTOR pathway.
5. Conclusion
mTOR pathway activation promotes tumorigenesis, and mTOR is a bona fide target in cancer. Inhibitors of mTOR such as rapamycin and its analogues are currently being evaluated in clinical trials for the treatment of many types of cancer. However, the therapeutic response to mTOR inhibitors in these trials has been variable, and prolonged administration of these drugs could be limited due to toxicities in patients. Preclinical and clinical studies also suggest that mTOR inhibition could result in feedback activation of Akt, which may limit the efficacy of these drugs as anticancer agents. Although the PI3K/Akt pathway is an important activator of mTOR, multiple signaling pathways regulate mTOR independently of Akt in response to mitogens, nutrient availability, and conditions that deplete intracellular energy, such as hypoxia. Targeting upstream components of these pathways may provide new therapeutic approaches for inhibiting mTOR in cancer. Drugs that activate AMPK, such as metformin and 2-DG, could have potential as anti-cancer agents because they are well tolerated and have favorable pharmacologic characteristics. Preclinical studies show that these AMPK activators can inhibit the mTOR pathway in cancer cells and inhibit tumor growth. However, these studies also suggested that the status of tumor suppressor genes, such as LKB1 and p53, and alterations in cancer cell glucose metabolism, might affect the clinical response to these agents. Future preclinical and clinical studies will be needed to determine if AMPK activators are more effective than classical inhibitors of mTOR such as rapamycin in the prevention or treatment of cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 5.Frech M, et al. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272(13):8474–81. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 6.Andjelkovic M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272(50):31515–24. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 7.Walker KS, et al. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J. 1998;331(Pt 1):299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balendran A, et al. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9(8):393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 9.Delcommenne M, et al. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95(19):11211–6. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch DK, et al. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18(56):8024–32. doi: 10.1038/sj.onc.1203258. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, et al. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279(39):41189–96. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 12.Hill MM, Feng J, Hemmings BA. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12(14):1251–5. doi: 10.1016/s0960-9822(02)00973-9. [DOI] [PubMed] [Google Scholar]
- 13.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275(12):8271–4. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 14.Inoki K, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 15.Manning BD, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10(1):151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 16.Garami A, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11(6):1457–66. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 17.Karbowniczek M, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167(7):976–82. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 18.Chan JA, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63(12):1236–42. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 19.El-Hashemite N, et al. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361(9366):1348–9. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- 20.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meikle L, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28(21):5422–32. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauktys A, et al. Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model. BMC Dermatol. 2008;8:1. doi: 10.1186/1471-5945-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacina KS, et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278(12):10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 24.Sancak Y, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Vander Haar E, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9(3):316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 26.Nave BT, et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Sekulic A, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60(13):3504–13. [PubMed] [Google Scholar]
- 28.Govindarajan B, et al. Tuberous sclerosis-associated neoplasms express activated p42/44 mitogen-activated protein (MAP) kinase, and inhibition of MAP kinase signaling results in decreased in vivo tumor growth. Clin Cancer Res. 2003;9(9):3469–75. [PubMed] [Google Scholar]
- 29.Han S, et al. Phosphorylation of tuberin as a novel mechanism for somatic inactivation of the tuberous sclerosis complex proteins in brain lesions. Cancer Res. 2004;64(3):812–6. doi: 10.1158/0008-5472.can-03-3277. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, et al. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121(2):179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Hardie DG, et al. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546(1):113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 32.Sanders MJ, et al. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403(1):139–48. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101(10):3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 36.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391(6663):184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 37.Ghaffar H, et al. LKB1 protein expression in the evolution of glandular neoplasia of the lung. Clin Cancer Res. 2003;9(8):2998–3003. [PubMed] [Google Scholar]
- 38.Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Cespedes M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62(13):3659–62. [PubMed] [Google Scholar]
- 40.Shaw RJ, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Wei C, et al. Suppression of Peutz-Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res. 2008;14(4):1167–71. doi: 10.1158/1078-0432.CCR-07-4007. [DOI] [PubMed] [Google Scholar]
- 42.Carretero J, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26(11):1616–25. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 43.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Hurley RL, et al. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280(32):29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 45.Woods A, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2(1):21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Kishi K, et al. AMP-Activated protein kinase is activated by the stimulations of G(q)-coupled receptors. Biochem Biophys Res Commun. 2000;276(1):16–22. doi: 10.1006/bbrc.2000.3417. [DOI] [PubMed] [Google Scholar]
- 47.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 48.Stahmann N, et al. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26(16):5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson KA, et al. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273(48):31880–9. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- 50.Memmott RM, et al. Phosphatidylinositol ether lipid analogues induce AMP-activated protein kinase-dependent death in LKB1-mutant non small cell lung cancer cells. Cancer Res. 2008;68(2):580–8. doi: 10.1158/0008-5472.CAN-07-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 52.Kimura N, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8(1):65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 53.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 54.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278(32):29655–60. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 56.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18(23):2893–904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280(11):9769–72. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- 58.Sofer A, et al. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25(14):5834–45. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeYoung MP, et al. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22(2):239–51. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, et al. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J Biol Chem. 2003;278(16):13663–71. doi: 10.1074/jbc.M300862200. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112(8):1223–33. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai SL, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173(2):279–89. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101(3):811–6. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Findlay GM, et al. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J. 2007;403(1):13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280(38):33076–82. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 66.Nobukuni T, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102(40):14238–43. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith EM, et al. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280(19):18717–27. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 68.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sekiguchi T, et al. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem. 2001;276(10):7246–57. doi: 10.1074/jbc.M004389200. [DOI] [PubMed] [Google Scholar]
- 70.Exton JH. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439(2):121–33. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 71.Fang Y, et al. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol. 2003;13(23):2037–44. doi: 10.1016/j.cub.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 72.Voss M, et al. Phospholipase D stimulation by receptor tyrosine kinases mediated by protein kinase C and a Ras/Ral signaling cascade. J Biol Chem. 1999;274(49):34691–8. doi: 10.1074/jbc.274.49.34691. [DOI] [PubMed] [Google Scholar]
- 73.Shi M, et al. Phospholipase D provides a survival signal in human cancer cells with activated H-Ras or K-Ras. Cancer Lett. 2007;258(2):268–75. doi: 10.1016/j.canlet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe H, et al. Essential role for phospholipase D2 activation downstream of ERK MAP kinase in nerve growth factor-stimulated neurite outgrowth from PC12 cells. J Biol Chem. 2004;279(36):37870–7. doi: 10.1074/jbc.M402610200. [DOI] [PubMed] [Google Scholar]
- 75.Sun Y, et al. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105(24):8286–91. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang Y, et al. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294(5548):1942–5. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22(25):3937–42. doi: 10.1038/sj.onc.1206565. [DOI] [PubMed] [Google Scholar]
- 78.Hornberger TA, et al. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103(12):4741–6. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foster DA. Targeting mTOR-mediated survival signals in anticancer therapeutic strategies. Expert Rev Anticancer Ther. 2004;4(4):691–701. doi: 10.1586/14737140.4.4.691. [DOI] [PubMed] [Google Scholar]
- 80.Chan S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23(23):5314–22. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 81.Duran I, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95(9):1148–54. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galanis E, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 83.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 84.Yee KW, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12(17):5165–73. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 85.Xie Z, et al. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117(7):952–62. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie Z, et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281(10):6366–75. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 87.Luna V, et al. Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects. Diabetologia. 2006;49(2):375–82. doi: 10.1007/s00125-005-0112-4. [DOI] [PubMed] [Google Scholar]
- 88.Musi N, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–81. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 89.Bolen S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–99. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 90.Evans JM, et al. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330(7503):1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zakikhani M, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 92.Dowling RJ, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 93.Huang X, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–21. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 94.Jones RG, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 95.Crighton D, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121–34. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 96.Buzzai M, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 97.Corton JM, et al. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 98.Lee MJ, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292(2):F617–27. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 99.Rattan R, et al. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280(47):39582–93. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 100.Elstrom RL, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 101.Buzzai M, et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24(26):4165–73. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 102.Dixon R, et al. AICA-riboside: safety, tolerance, and pharmacokinetics of a novel adenosine-regulating agent. J Clin Pharmacol. 1991;31(4):342–7. doi: 10.1002/j.1552-4604.1991.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 103.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87(5):1990–5. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 104.Leung JM, et al. An initial multicenter, randomized controlled trial on the safety and efficacy of acadesine in patients undergoing coronary artery bypass graft surgery. SPI Research Group. Anesth Analg. 1994;78(3):420–34. doi: 10.1213/00000539-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Corradetti MN, et al. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18(13):1533–8. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhong D, et al. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer. 2006;53(3):285–94. doi: 10.1016/j.lungcan.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 107.Mohanti BK, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35(1):103–11. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 108.Maschek G, et al. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64(1):31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 109.Varshney R, Dwarakanath B, Jain V. Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. Int J Radiat Biol. 2005;81(5):397–408. doi: 10.1080/09553000500148590. [DOI] [PubMed] [Google Scholar]
- 110.Heminger K, et al. Altered gene expression induced by ionizing radiation and glycolytic inhibitor 2-deoxy-glucose in a human glioma cell line: implications for radio sensitization. Cancer Biol Ther. 2006;5(7):815–23. doi: 10.4161/cbt.5.7.2812. [DOI] [PubMed] [Google Scholar]
- 111.Lin X, et al. 2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63(12):3413–7. [PubMed] [Google Scholar]
- 112.Zhu Z, et al. 2-Deoxyglucose as an energy restriction mimetic agent: effects on mammary carcinogenesis and on mammary tumor cell growth in vitro. Cancer Res. 2005;65(15):7023–30. doi: 10.1158/0008-5472.CAN-05-0453. [DOI] [PubMed] [Google Scholar]
- 113.Jiang W, Zhu Z, Thompson HJ. Modulation of the activities of AMP-activated protein kinase, protein kinase B, and mammalian target of rapamycin by limiting energy availability with 2-deoxyglucose. Mol Carcinog. 2008;47(8):616–28. doi: 10.1002/mc.20425. [DOI] [PubMed] [Google Scholar]
- 114.Wangpaichitr M, et al. Intrinsically lower AKT, mammalian target of rapamycin, and hypoxia-inducible factor activity correlates with increased sensitivity to 2-deoxy-D-glucose under hypoxia in lung cancer cell lines. Mol Cancer Ther. 2008;7(6):1506–13. doi: 10.1158/1535-7163.MCT-07-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castillo SS, et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64(8):2782–92. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 116.Cool B, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3(6):403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Goransson O, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282(45):32549–60. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guigas B, et al. Beyond AICA riboside: In search of new specific AMP-activated protein kinase activators. IUBMB Life; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]